Redox responsive amphiphilic copolymer as well as preparation method and application thereof
An amphiphilic copolymer and a responsive technology, which can be used in drug combinations, pharmaceutical formulations, organic active ingredients, etc., can solve the problems of limited tumor treatment effect and inability to meet the needs of use, and achieve simple preparation methods and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: Preparation of Compound III (N,N'-bis(acryloyl)cystamine):
[0067]
[0068] Dissolve 2.5g of cystamine hydrochloride (compound II) and 1.8g of NaOH in 15.5ml of deionized water, and add a solution of acryloyl chloride in dichloromethane (3.3ml of acryloyl chloride in 3.3ml of anhydrous di Chloromethane), after the dropwise addition, remove the ice bath, react at room temperature for 6 hours, finish the reaction, add 200ml dichloromethane to the reaction solution for extraction, extract three times, combine the organic phases, and the organic phases are distilled under reduced pressure and vacuum-dried to obtain a white powdery substance , that is, compound III (N,N'-bis(acryloyl)cystamine).
[0069] Tested: 1 H NMR (600MHz, CDCl 3 ): δ6.68(s,1H), 6.34(dd, J=17.0,1.5Hz,1H), 6.24(dd,J=17.0,10.2Hz,1H), 5.69(dd,J=10.2,1.5Hz, 1H), 3.69 (q, J=6.4Hz, 2H), 2.90 (t, J=6.5Hz, 2H).
Embodiment 2
[0070] Embodiment 2: the preparation of compound IV (hyperbranched polyamidoamine PAAs):
[0071]
[0072] Dissolve 0.642g of compound III and 0.592g of anhydrous calcium chloride in 15ml of aqueous methanol (the volume ratio of methanol / water is 3 / 1). Under the protection of nitrogen, the temperature of the reaction solution is raised to 50°C, and 0.16ml of 1-(2-Aminoethyl)piperazine (AEPZ), after the dropwise addition is completed, keep the reaction at 50°C for 36 hours, then add 0.35ml 1-(2-Aminoethyl)piperazine (AEPZ) dropwise again, continue Insulate the reaction for 4 hours, end the reaction, add dilute hydrochloric acid to adjust the pH of the reaction system to 3-4, then add the reaction solution into a dialysis bag (MWcutoff=1000), dialyze for 24 hours, centrifuge to remove the precipitate, and freeze-dry the solution to obtain a light yellow viscous Thickness, that is, compound IV (hyperbranched polyamidoamine PAAs).
[0073] Tested: 1 H NMR (600MHz,D 2 O)δ3.60...
Embodiment 3
[0074] Embodiment 3: Preparation of redox-responsive amphiphilic hyperbranched polyamidoamine-gambogic acid copolymer PAG:
[0075]
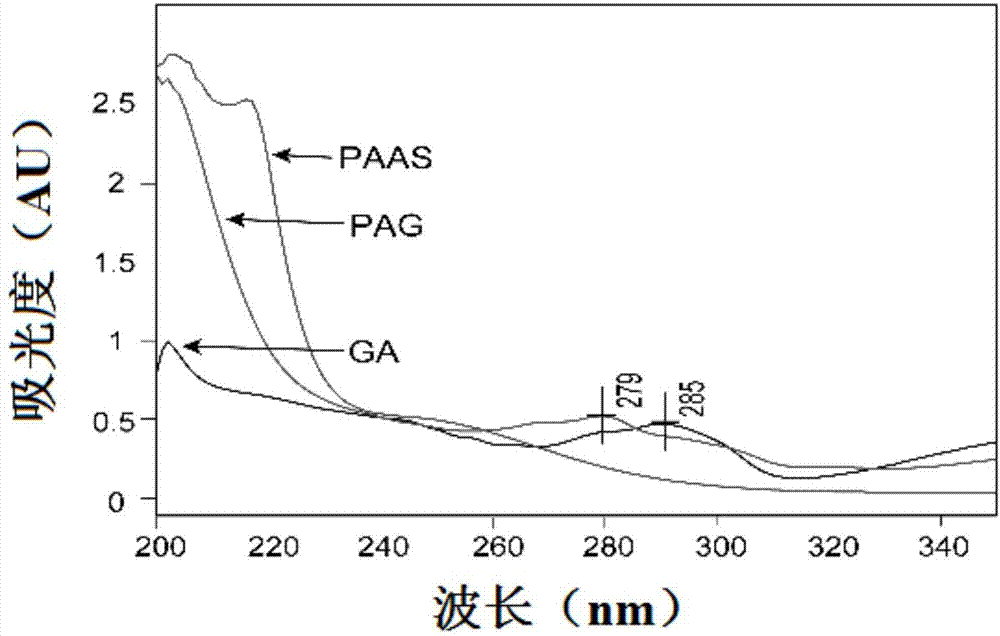
[0076]Dissolve 0.5g gambogic acid (GA) and 0.1g 4-lutidine (DMAP) in 10ml of anhydrous dichloromethane, and add 0.3g 1-ethyl-(3-dimethyl Aminopropyl) carbodiimide (EDC), stirred in an ice bath for 1 hour, then added 1.5 g of compound IV, and reacted at room temperature for 24 hours. hours, centrifuged to remove the precipitate, and the solution was freeze-dried to obtain a yellow viscous substance, that is, a hyperbranched polyamidoamine-gambogic acid copolymer (PAG for short).
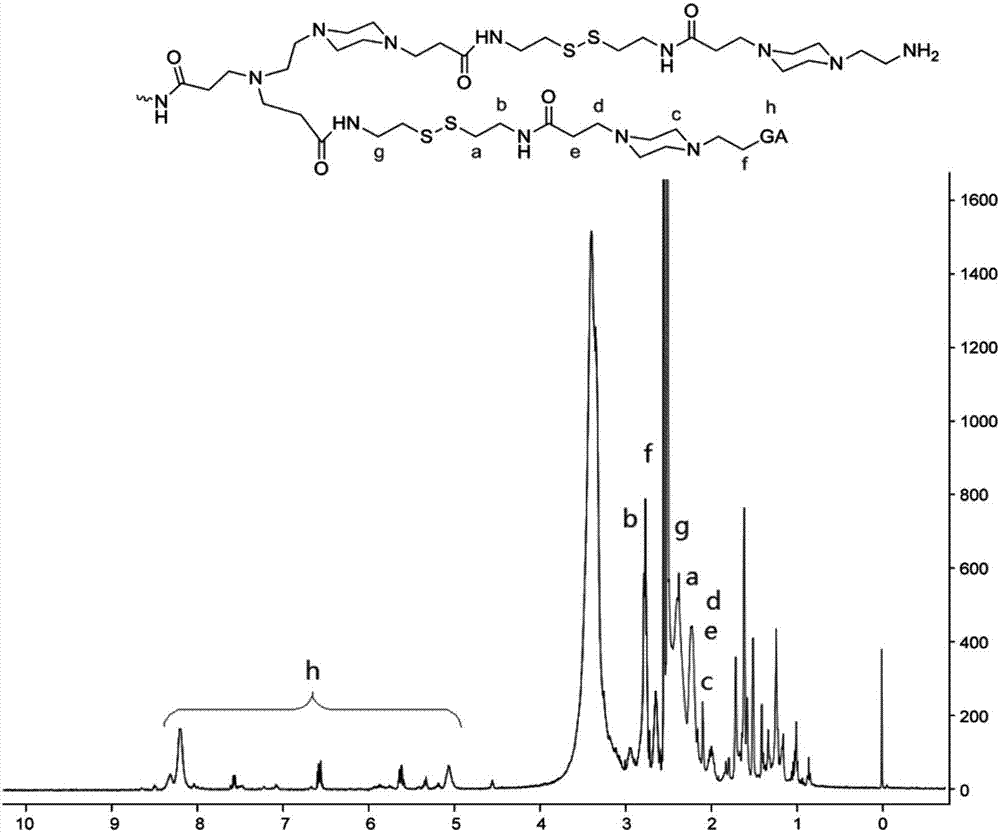
[0077] figure 1 is the NMR spectrum of PAG ( 1 H-NMR (DMSO-d)), among the figure, δ5.0-9.0ppm belongs to the characteristic peak (h) of gambogic acid, and simultaneously the characteristic peak (a-f) of CBA and 1-(2-aminoethyl) piperazine also Marked in the hydrogen spectrum, proves that the compound that makes is required hyperbranched polyamidoamine-gambogic aci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| surface potential | aaaaa | aaaaa |
| surface potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com