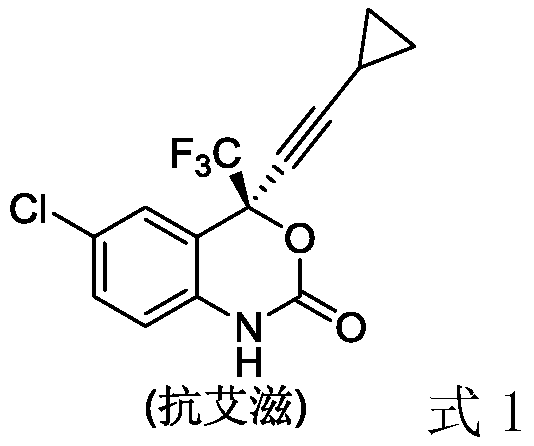
A method for the synthesis of chiral fluorine-containing propargylamine derivatives by biomimetic catalytic asymmetric hydrogenation
A biomimetic catalysis, asymmetric technology, applied in the preparation of organic compounds, organic chemical methods, preparation of amino compounds, etc., to achieve the effects of high chemical selectivity and enantioselectivity, specific products, and convenient separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: optimization of conditions
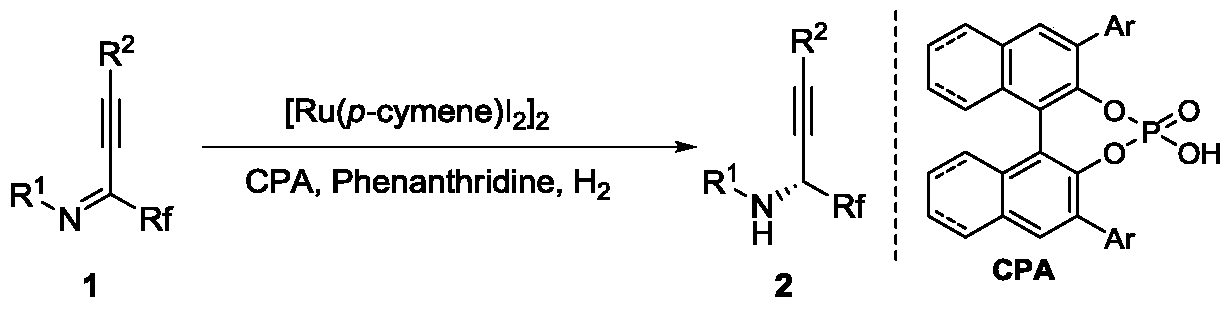
[0036] Add weighed (4-isopropyltoluene) ruthenium iodide dimer (0.001 mmol, 1.0 mg) and chiral phosphoric acid (0.0004 mmol), 8-methylphenanthridine (0.02 mg mol, 3.8 mg), fluoroalkynyl imine (0.2 mmol), 1,2-dichloroethane 2.0 ml, stirred for 2-5 minutes. Then put the ampule bottle into a stainless steel autoclave, feed hydrogen 1000psi, and react at room temperature for 20-48 hours. Slowly release hydrogen, remove the solvent with a rotary evaporator, and then directly column chromatography (the volume ratio of eluent petroleum ether and ethyl acetate is 30:1) to separate the pure product, the reaction formula and the ligand are as follows:
[0037]
[0038] The yield is the isolated yield, and the enantiomeric excess of the product is determined by chiral liquid chromatography, see Table 1.
[0039] Table 1. Screening of solvents and chiral phosphoric acids
[0040]
[0041] [a] Reaction conditions: 1a (0.1mmol), CPA...
Embodiment 2
[0042]Example 2: Synthesis of Chiral Fluorine-Containing Propargylamine Derivatives by Biomimetic Catalytic Asymmetric Selective Hydrogenation
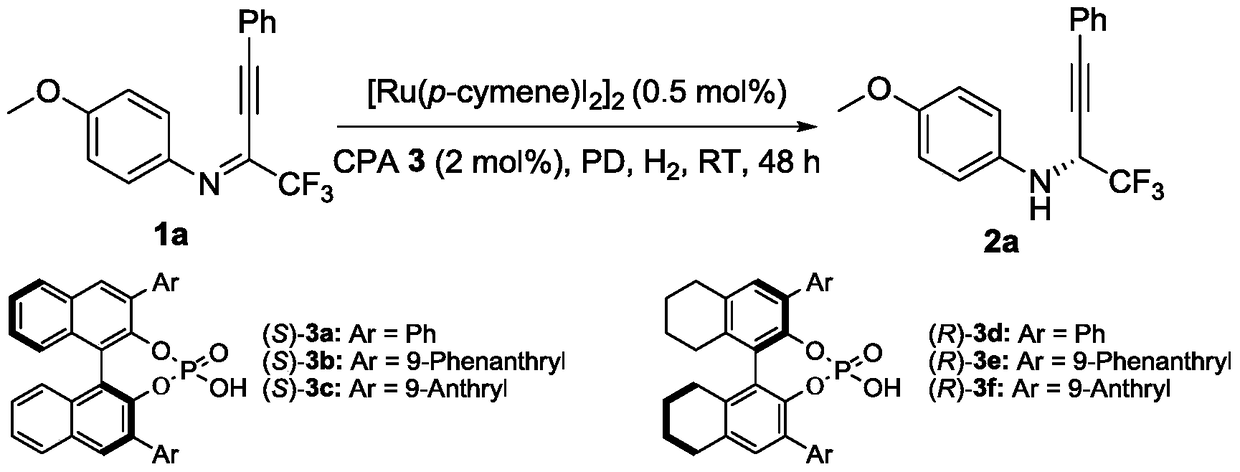
[0043] Add weighed (4-isopropyltoluene) ruthenium iodide dimer (0.001 mmol, 1.0 mg) and chiral phosphoric acid ((R)-3f, 0.0004 mmol) to the ampoule, 8-methyl Kiphenanthridine (0.02 mmol, 3.8 mg), fluoroalkynyl imine (0.2 mmol), 1,2-dichloroethane 2.0 ml, stirred for 2-5 minutes. Then put the ampule bottle into a stainless steel autoclave, feed hydrogen 1000psi, and react at room temperature for 20-48 hours. Slowly release hydrogen, remove the solvent with a rotary evaporator and then directly column chromatography (the volume ratio of eluent sherwood oil and ethyl acetate is 30:1) to separate and obtain pure product, the reaction formula is as follows:
[0044]
[0045] (R)-4-Methoxy-N-(1,1,1-trifluoro-4-phenylbut-3-yn-2-yl)aniline(2a): 95% yield, pale yellow oil, R f =0.60(petroleum ether / ethyl acetate=30 / 1), 95%ee, [α] 20 D =...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com