Chiral crown ether column capable of effectively splitting amino acid at normal temperature
A technology for amino acids and crown ethers, applied in the field of chromatographic separation columns, can solve problems such as harsh temperature conditions, unfavorable daily applications, and poor chiral resolution of mixed amino acids, achieving low cost and high reproducibility for column preparation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
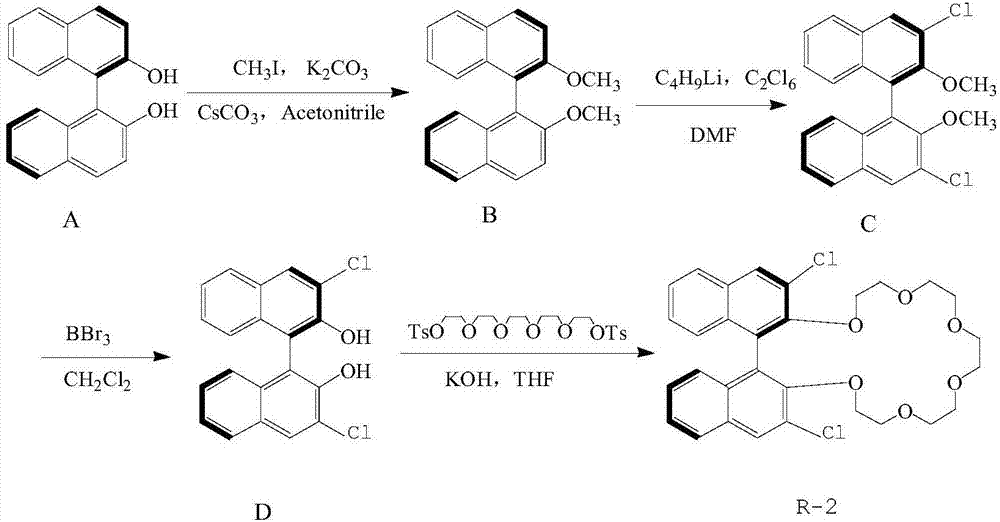
[0027] The synthetic steps of R‐(3,3'‐chloro‐1,1'‐binaphthyl)‐20‐crown‐6, such as figure 1 Shown:
[0028] in N 2 Under protection, add 150ml acetone to a 500mL round bottom flask, then add 8g R‐binaphthol in turn, add dry and fine potassium carbonate powder K 2 CO 3 (20g, 0.2mol), stirred evenly and heated to 70°C, slowly added iodomethane (5.1mL, 0.10mol) dropwise through a constant pressure funnel, refluxed for 12h and then detected by TCL. , (eluent, acetone:cyclohexane=1:10, with distilled water, with dichloromethane extraction, with anhydrous NaSO 4 The solid was dried and filtered, and the solvent was removed under reduced pressure, and the residue was purified by a silica gel column (eluent, acetone:petroleum ether=1:40) and passed through the column. Obtained white crystal B;
[0029] in N 2 Under protection, dissolve 4g of B into 150mL ether solution, then add 32.1mL of n-butyllithium and 5.6mL of tetramethyldiethylamine, stir at 25°C for 8h, cool the reactant ...
Embodiment 2
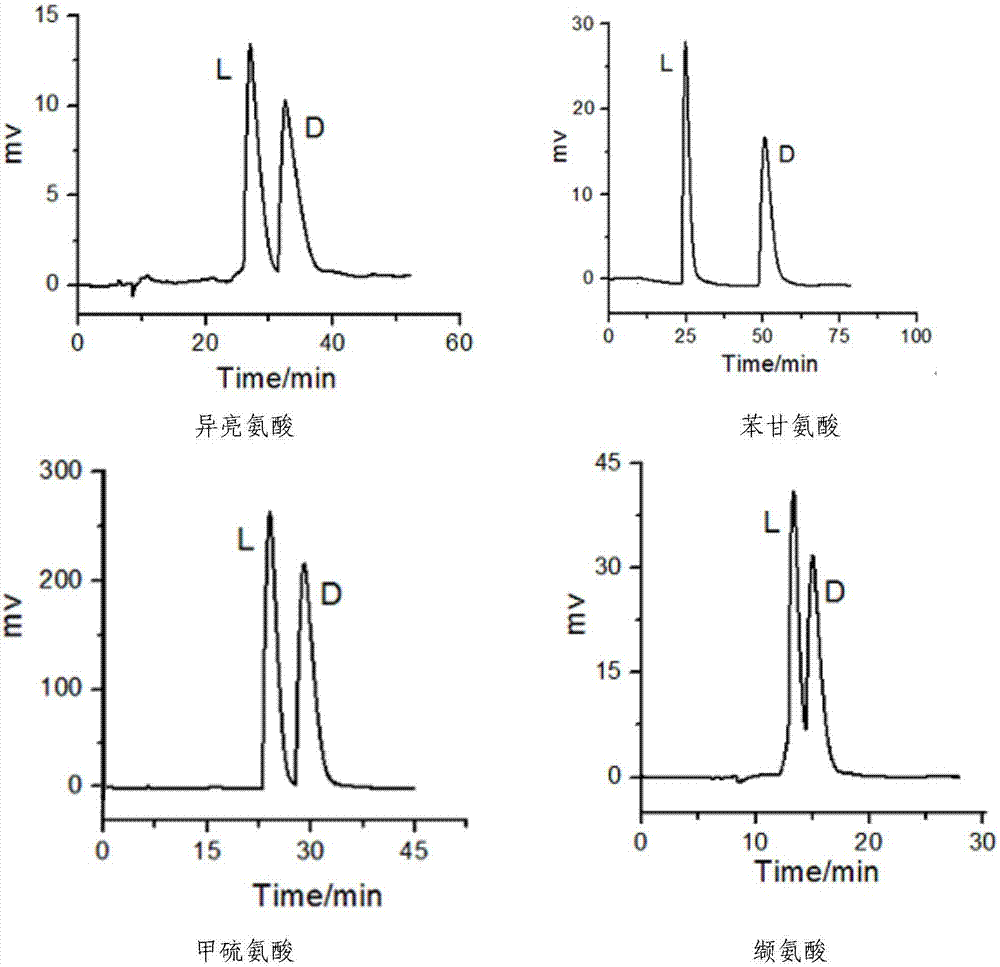
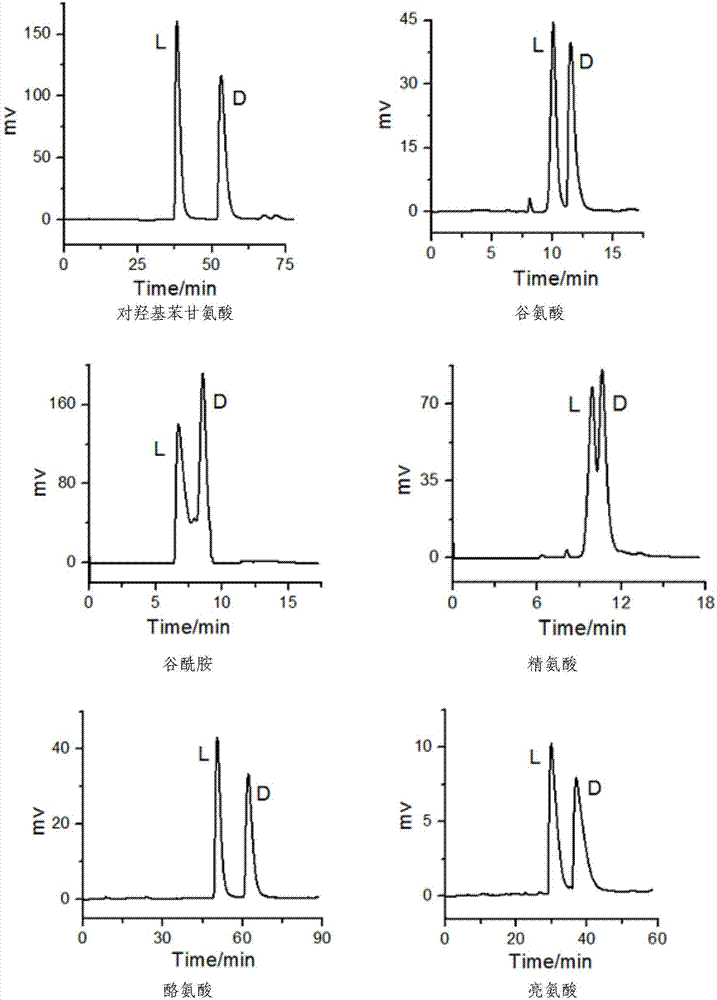
[0039] After R‐(3,3'‐dibromo‐1,1'‐binaphthyl)‐20‐crown‐6 chiral stationary phase was used for 240 hours under the above chromatographic experimental conditions, the retention factor K of each α‐amino acid, The separation factor α and resolution Rs remain basically unchanged, indicating that the R-(1,1'-dinaphthyl)-20-crown-6 chiral stationary phase is very stable and can be used for a long time under the above chromatographic conditions .
Embodiment 3
[0041] The mobile phase is the perchloric acid solution of pH=2, V (flow rate)=0.4mL / min, T (column temperature)=25 ℃ of conditions that the detection wavelength is 210nm, the present invention R-(3,3'-dibromo Base‐1,1'‐binaphthyl)‐20‐crown‐6 chiral crown ether column compared with Japanese commercial column CROWNPAKCR(+)CSP, the typical comparison spectrum is as follows image 3 shown. At room temperature, histidine and valine, which cannot be resolved in the commercial column, were separated from the R‐(3,3'‐dibromo‐1,1'‐binaphthyl)‐20‐crown‐6 crown ether column can be effectively resolved, and serine can be better resolved in the chromatographic column of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com