Preparation method and application of a kind of anti-chronic inflammation mesenchymal stem cells
A technology of mesenchymal stem cells and chronic inflammation, applied in the field of anti-chronic inflammation application and mesenchymal stem cell injection for anti-chronic inflammation, can solve the problems of low efficiency and uncertain treatment effect, achieve optimal treatment plan, enhance anti-inflammatory and anti-inflammatory effects. The effect of inflammation, the effect of easy quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1. Preparation of umbilical cord mesenchymal stem cells
[0028]Operate in an ultra-clean workbench, take fresh full-term healthy fetal umbilical cord, wash it with normal saline containing 2 times 100U / mL penicillin and streptomycin to remove blood stains, peel off Walton’s jelly, and cut it into pieces less than 1 cubic millimeter Shape, spread evenly, slowly add 10mL serum-free medium, and culture in a 37°C, 5% carbon dioxide, saturated humidity incubator. After the primary cells were cultured for 7 days, the serum-free medium was replaced and cultured until the cells reached 70% confluence, the serum-free medium was removed, and trypsin-sodium edetate ( EDTA) for 1 min, the umbilical cord MSCs shrank and detached from the bottle wall. At this time, the previously removed culture supernatant was added to neutralize the trypsin-EDTA solution, and gently blown with a pipette, and the umbilical cord MSC suspension was transferred to a 50 mL centrifuge. Tube. C...
Embodiment 2
[0031] Example 2. LPS hypoxic pretreatment of umbilical cord mesenchymal stem cells
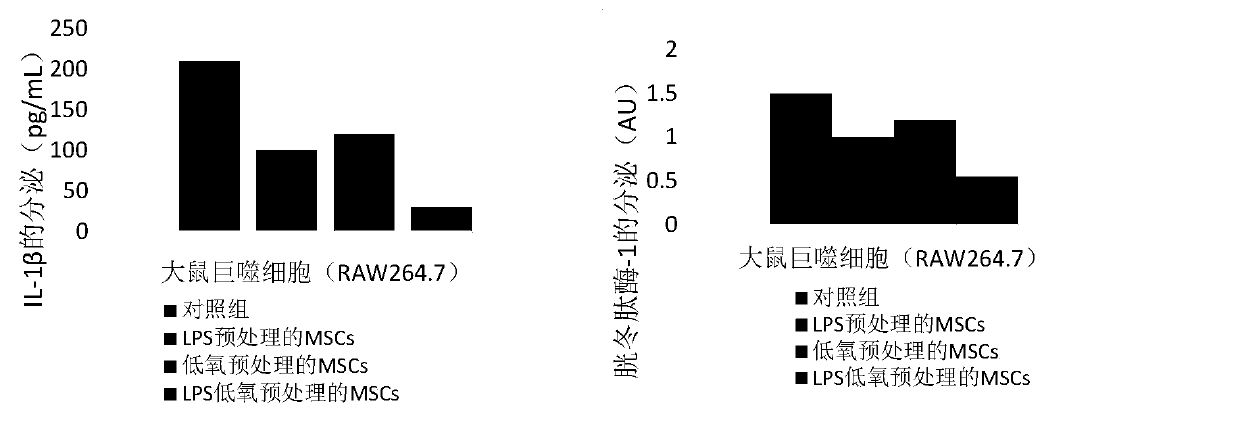
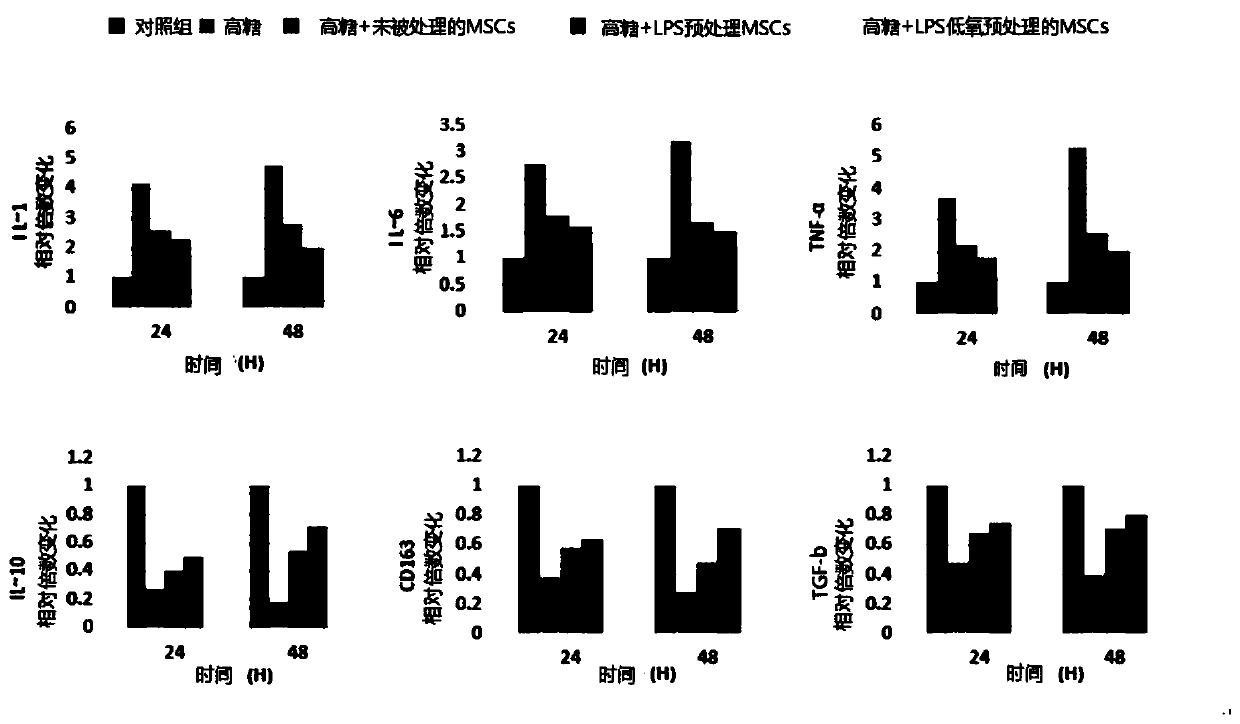
[0032] The anti-chronic inflammation mesenchymal stem cells are LPS hypoxic pretreated mesenchymal stem cells, and the preparation method of the anti-chronic inflammation mesenchymal stem cells includes the following steps: a) culturing the mesenchymal stem cells to 70-80 % fusion, remove the culture medium in the mesenchymal stem cell culture dish, wash the mesenchymal stem cells with phosphate buffer; b) add serum-free medium containing 50-200ng / mL lipopolysaccharide (LPS); then c) Adjusting the hypoxic state to an oxygen content of 5-10%, and culturing the mesenchymal stem cells in a hypoxic state for 16-18 hours, measuring the number of cultured mesenchymal stem cells, if the number of mesenchymal stem cells can reach 5 ×10 5 ~2×10 6 cells / mL, indicating effective LPS and hypoxia pretreated mesenchymal stem cells.
[0033] (1)
[0034] Inoculate 1.5×10 per 15 cm cell culture dish 6 U...
Embodiment 3
[0039] Example 3. Preparation of Mesenchymal Stem Cell Injection against Chronic Inflammation
[0040] An anti-chronic inflammation mesenchymal stem cell injection, which includes the following components: the quantity is 5×10 5 ~2×10 6 LPS hypoxic pretreated mesenchymal stem cells per mL, the mass volume ratio is 1-5% human serum albumin, the mass volume ratio is 1-8% mannitol injection, and the mass volume ratio is 1-5% compound Vitamin injection, 50% glucose injection with a mass volume ratio of 1-5% and normal saline as the balance, wherein the mesenchymal stem cells against chronic inflammation are lipopolysaccharide and hypoxic pretreated mesenchymal stem cells.
[0041] For example, prepare 100mL of anti-chronic inflammation mesenchymal stem cell injection, which includes 5mL of human serum albumin stock solution (the final concentration of mass volume ratio is 1%), 2mL of mannitol injection, 3mL of compound vitamin injection, and 50% glucose injection. MSCs pretreate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com