Coumarin-based relay-responsive fluorescent molecular probe and its preparation method and use
A fluorescent molecular probe, coumarin-based technology, applied in the field of chemical fluorescence sensing materials, can solve the problems of difficult rapid and effective detection, poor anti-interference, complicated operation, etc., and achieves easy control of synthesis conditions and fast response time. , post-processing simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
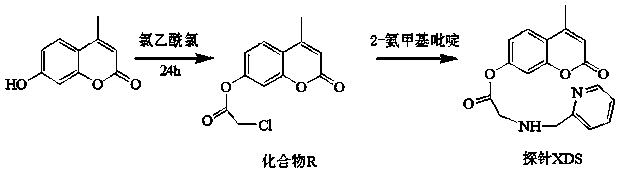
[0040] Step 1, preparation of compound R: Dissolve 0.5g (3mmol) 4-methylumbelliferone and 0.1mL triethylamine in 25mL anhydrous dichloromethane in a round-bottomed flask, and cool the mixture down in an ice bath to 0°C, add 0.24mL (3mmol) chloroacetyl chloride dropwise to the above solution, and then 2 The reaction was stirred under protection for 6h. After the reaction, add 100mL of distilled water, and then use CH 2 Cl 2 Extract three times. Combine the organic layers with anhydrous Na 2 SO 4 Dry, filter and distill off the solvent under reduced pressure to give the product as a white solid. The crude product was purified by recrystallization in ethanol to obtain 0.47 g of compound R with a yield of 63%.
[0041] Step 2. Preparation of fluorescent molecular probe XDS: 0.45g (4mmol) 2-aminomethylpyridine, 0.34g (4mmol) NaHCO 3 , 0.55 g (4 mmol) K 2 CO 3 and 0.64g (4mmol) KI were placed in a round-bottomed flask, and 1.006g (4mmol) of compound R was weighed and dissol...
Embodiment 2
[0043] Step 1, preparation of compound R: Dissolve 1 g (6 mmol) 4-methylumbelliferone and 0.3 mL triethylamine in 35 mL anhydrous dichloromethane in a round-bottomed flask, and cool the mixture in an ice bath to to 0°C, add 0.48g (6mmol) chloroacetyl chloride dropwise to the above solution, and then 2 The reaction was stirred for 9h under protection. After the reaction, add 100mL of distilled water, and then use CH 2 Cl 2 Extract three times. Combine the organic layers with anhydrous Na 2 SO 4 Dry, filter and distill off the solvent under reduced pressure to give the product as a white solid. The crude product was purified by recrystallization in ethanol to obtain 0.87 g of compound R with a yield of 70%.
[0044] Step 2. Preparation of fluorescent molecular probe XDS: 1.35g (12mmol) 2-aminomethylpyridine, 1.02g (12mmol) NaHCO 3 , 1.65 g (12 mmol) K 2 CO 3 and 1.86g (12mmol) KI were placed in a round-bottomed flask, and 3.018g (12mmol) of compound R was weighed and di...
Embodiment 3
[0046] Step 1, preparation of compound R: 1.6g (9mmol) 4-methylumbelliferone and 0.5mL triethylamine were dissolved in 50mL anhydrous dichloromethane and placed in a round bottom flask, and the mixture was cooled down in an ice bath to 0°C, add 0.72mL (9mmol) chloroacetyl chloride dropwise to the above solution, and then 2 The reaction was stirred for 12h under protection. After the reaction, add 100mL of distilled water, and then use CH 2 Cl 2 Extract three times. Combine the organic layers with anhydrous Na 2 SO 4 Dry, filter and distill off the solvent under reduced pressure to give the product as a white solid. The crude product was purified by recrystallization in ethanol to obtain 1.27 g of compound R with a yield of 68%.
[0047] Step 2. Preparation of fluorescent molecular probe XDS: 2.2g (20mmol) 2-aminomethylpyridine, 3.5g (20mmol) NaHCO 3 , 2.8g (20mmol) 2.5g K 2 CO 3 (20mmol) and 3.4g (20mmol) KI were placed in a round-bottomed flask, and 1.006g (20mmol) o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com