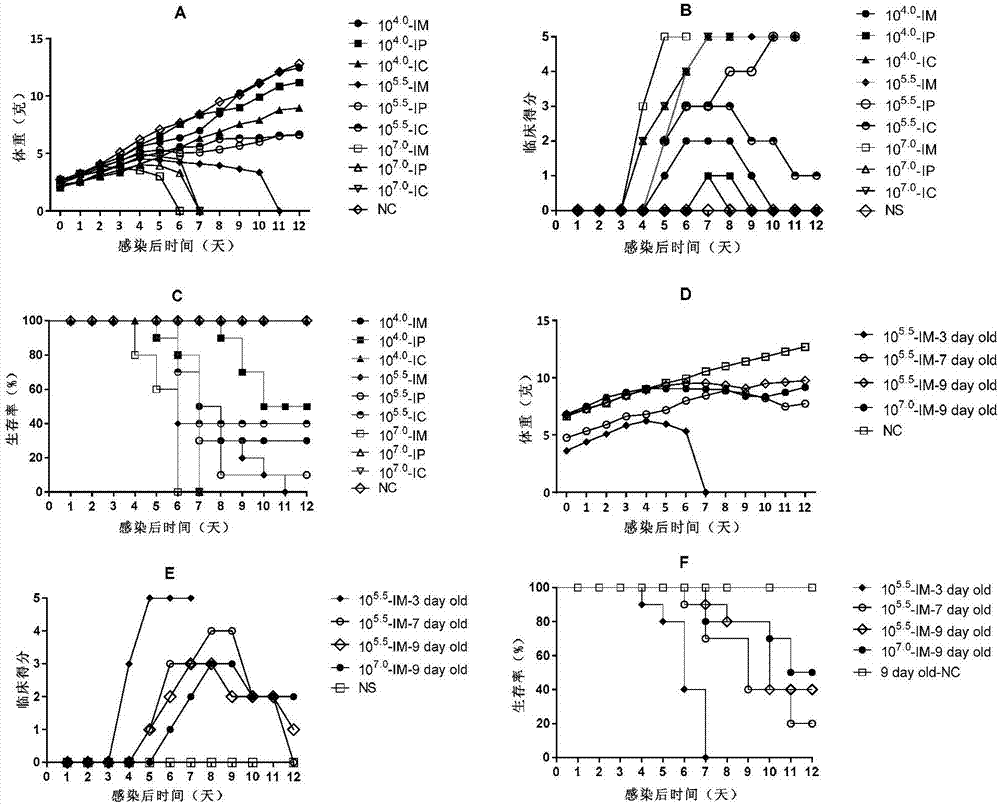
Coxsackievirus A6 type infected animal model as well as preparation method and application thereof
A technology for coxsackie virus and infecting animals, applied in the biological field, can solve the problems of lack of stable animal infection models, constraints on the development of anti-CVA6 drugs and vaccines, and unclear pathogenesis of hand-foot-mouth disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1, Isolation and Identification of Coxsackievirus Type A6 (CVA6) Strain WF057R
[0044] In 2015, the inventor isolated a Coxsackievirus type A6 (Coxsackievirus) strain from the feces of a hand-foot-mouth child in Shandong Province, and named the strain WF057R. Coxsackievirus type A6 (Coxsackievirus) strain WF057R has been preserved in the General Microbiology Center of China Committee for the Collection of Microbial Cultures (CGMCC for short) on December 26, 2016. The address is: Courtyard 3, No. 1 Beichen West Road, Chaoyang District, Beijing No.), the deposit number is CGMCC No.13393.
Embodiment 2
[0045] Example 2, Utilizing Coxsackie A6 Virus Strain WF057R to Prepare CVA6 Infected Animal Model
[0046] 1. Cultivation of WF057R
[0047] RD cells were inoculated in medium 1 (medium 1 is the liquid obtained by adding fetal bovine serum, penicillin and streptomycin to MEM maintenance solution, wherein the mass percentage concentration of fetal bovine serum is 10%, and the mass percentage of penicillin Concentration is 1%, the mass percent concentration of streptomycin is 1%), in temperature is 37 ℃, 5% concentration CO 2 cultured in a cell incubator to obtain RD cell culture medium. The Coxsackie A6 virus strain WF057R of Example 1 was inoculated on the RD cells in the RD cell culture medium, and when the cells had a cytopathic effect (Cytopathic effect, CPE) area exceeding 80%, the cell culture medium was collected to obtain CVA6 strain WF057R virus liquid, quantify virus by limiting dilution method in 96-well plate, and measure virus TCID 50 Afterwards, it was aliquot...
Embodiment 3
[0079] Example 3. The antiserum produced by the active CVA6 vaccine using inactivated WF057R can protect mice and treat diseases caused by CVA6
[0080] 1. Preparation of inactivated CVA6 vaccine
[0081] The diluted CVA6 strain WF057R virus liquid (10 5 TCID 50 / ml) was diluted with formalin at a ratio of 1:4000 (V / V) to obtain a virus dilution, and the virus dilution was incubated at 37°C for 72 hours to obtain an inactivated virus solution; the inactivated virus mixed with complete Freund's adjuvant in equal volumes to make a complete emulsion, which is the CVA6 vaccine, and the content of inactivated virus in the CVA6 vaccine is 5×10 4 TCID 50 / ml. The infectious titer of CVA6 vaccine was detected by micro titration method to observe its inactivation effect.
[0082] Virus micro titration method: 1×10 per well 4 Cell / 100 μ l culture fluid (the cell in this culture fluid is RD cell, and substratum is the substratum 1 of embodiment 2) RD cell is inoculated on 96 wells ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com