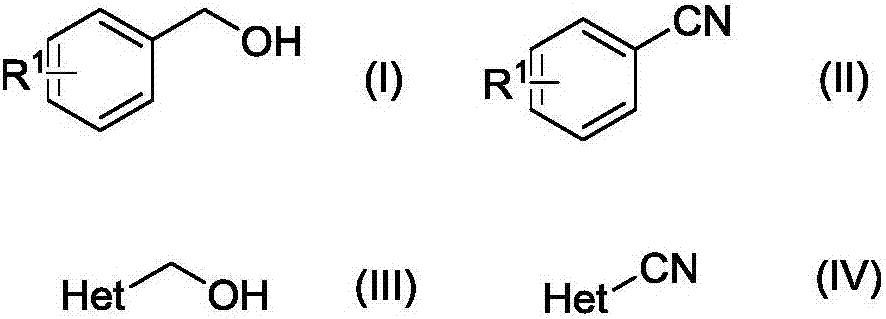
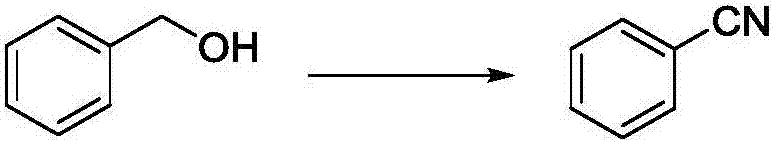Method for preparing nitrile from primary alcohol
A primary alcohol and catalyst technology, which is applied in the field of catalyzing primary alcohols to prepare nitriles, can solve the problems of harsh reaction conditions, high cost, and high reaction temperature, and achieve the effects of strong substrate applicability, convenient operation, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
[0031] In a 150mL thick-walled pressure-resistant tube equipped with magnetic stirring, under an air atmosphere, add benzyl alcohol (that is, the R in the structural formula (I) 1 H) 1.0mmol (108.1mg), ammonia water (1.6mol / L) 5.0mL, cuprous iodide 5mol% (9.5mg), TEMPO 5mol% (7.8mg), react at 100°C for 15h, after the reaction is completed, The reaction solution was cooled to room temperature and extracted with ethyl acetate (3×5.0 mL). The organic layers were combined and concentrated in vacuo to remove ethyl acetate to obtain a crude product. The crude product was purified by column chromatography (petroleum ether: ethyl acetate = 10:1) to obtain the pure target product. 94.9mg was obtained, the yield was 92%.
[0032] Proton nuclear magnetic resonance spectrum: 1H NMR (500MHz, CDCl3): δ7.61–7.53 (m, 3H), 7.43 (t, J=7.8Hz, 2H).
[0033] Carbon NMR spectrum: 13C NMR (125MHz, CDCl3): δ132.5, 131.8, 128.9, 118.5, 112.1.
Embodiment 2
[0035]
[0036] The reactant used is p-methyl benzyl alcohol (i.e. R in the structural formula (I) 1 For para-position COOH) 1.0mmol (152.1mg), experimental method and step are with embodiment 1, ammoniacal liquor (1.8mol / L) 3.0mL, the consumption of catalyzer cuprous bromide is 8mol% (11.5mg), the consumption of TEMPO is 5mol% (7.8mg), the reaction temperature was 120°C, and the reaction time was 24h. The crude product was purified by column chromatography (petroleum ether: ethyl acetate = 5:1) to obtain the pure target product, 129.4mg, yield 88% .
[0037] Proton nuclear magnetic resonance spectrum: 1H NMR (500MHz, CDCl3): δ8.23 (d, J=8.4Hz, 1H), 7.81 (d, J=8.4Hz, 1H).
[0038] Carbon NMR spectrum: 13C NMR (125MHz, CDCl3): δ169.1, 133.1, 132.4, 130.6, 117.7, 117.2.
Embodiment 3
[0040]
[0041] The reactant used is o-methoxybenzyl alcohol (that is, R in the structural formula (I) is ortho-position OCH 3 ) 1.0mmol (138.2mg), experimental method and step are with embodiment 1, ammoniacal liquor (1.7mol / L) 5.0mL, the consumption of catalyzer cuprous chloride is 8mol% (7.9mg), and the consumption of TEMPO is 8mol% (12.5 mg), the reaction temperature was 120°C, and the reaction time was 24 h. The crude product was purified by column chromatography (petroleum ether: ethyl acetate = 10:1) to obtain the pure target product, 123.9 mg, yield 93%.
[0042] Proton NMR spectrum: 1H NMR (500MHz, CDCl3): δ7.57–7.51(m,2H), 7.04–6.95(m,2H), 3.93(s,3H).
[0043] Carbon NMR spectrum: 13C NMR (125MHz, CDCl3): δ161.2, 134.3, 133.7, 120.7, 116.4, 111.3, 101.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com