Method for synthesizing phthaloyl amlodipine
A technology of phthaloyl ammonium chloride and phthalimide ethoxy, which is applied in the field of drug synthesis, can solve the problems of delayed drug action, slow binding and dissociation speed, etc., and achieve excellent quality, Fully responsive and easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] The technical solutions of the present invention will be further described below in conjunction with specific examples, and the examples should not be construed as limitations on the technical solutions.
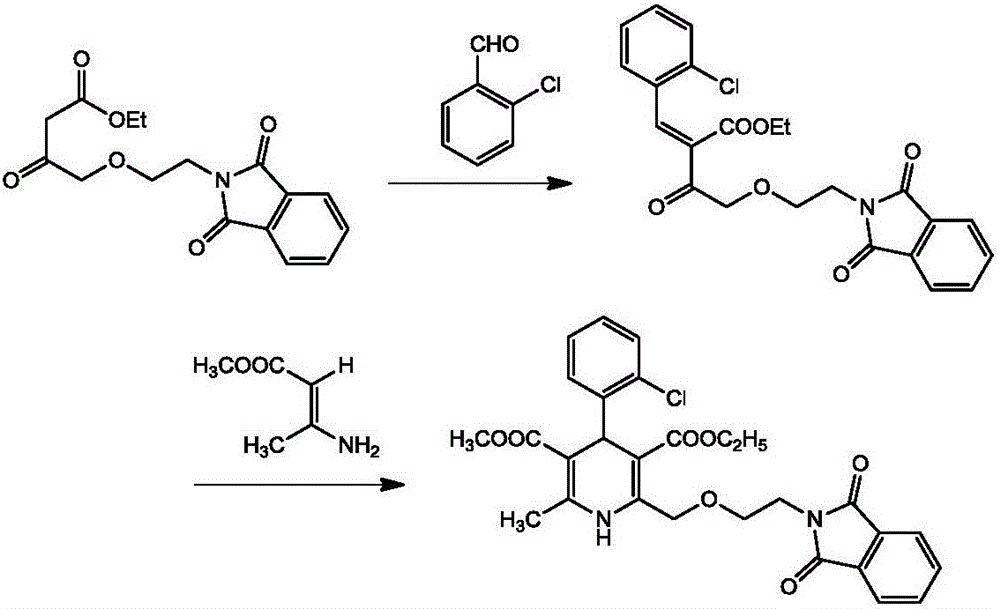
[0027] A kind of synthetic method of phthaloyl amlodipine, comprises the following steps:
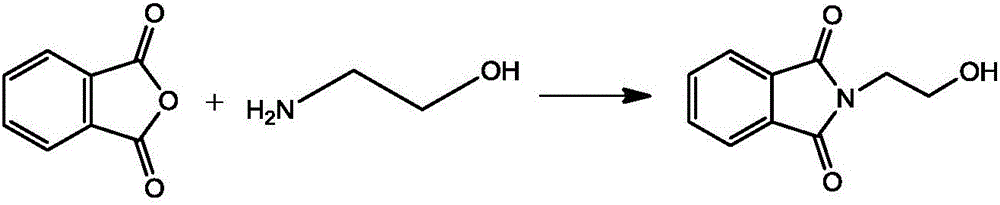
[0028] (1) Synthesis of N-hydroxyethylphthalimide
[0029]
[0030] Add 200g of phthalic anhydride and 600ml of toluene to the reaction flask equipped with a water separator, raise the temperature, add 100g of ethanolamine dropwise, reflux until the reaction is complete, cool and filter, dry at 80-90°C to obtain 247.6g of N-hydroxyethyl o-phthalate Dicarboximide, the yield is 96%.
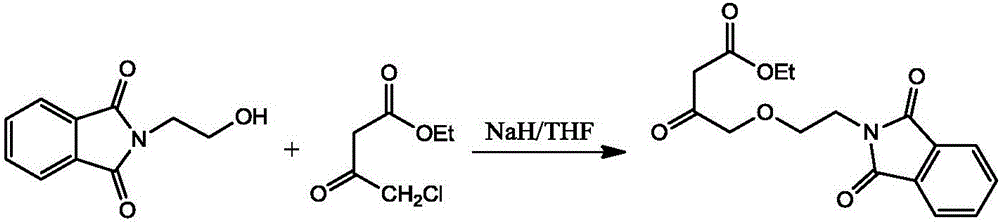
[0031] (2) Synthesis of ethyl-4-(2-phthalimidoethoxy) ethyl acetoacetate
[0032]
[0033] a. Put 2400ml tetrahydrofuran into the reaction bottle A, stir and cool to below -10°C, add 750g N-hydroxyethylphthalimide and 345g NaH in turn (while cooling, stir at a low speed, N-hydroxyethylphthal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com