Composition and reserve of bone filling material as well as preparation methods and applications of composition and reserve
A filling material and composition technology, used in tissue regeneration, medical science, prosthesis, etc., can solve the problems of inability to promote osteogenesis, high hardness, difficult placement, etc., to promote osteogenic differentiation and osteoblast function, Good shape and easy to shape effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
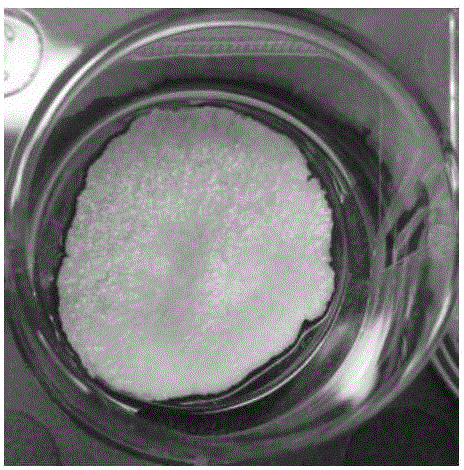
[0060] (1) The finished Bio-Oss bone meal (purchased from Geistlich Pharma AG) was ground into powder under sterile conditions, and the ground powder particles were observed under an environmental scanning electron microscope, and the particle size was about 10um-200um. The particle size is just suitable for mixing to make a scaffold material with uniform elasticity and hardness, and to form gaps of appropriate size to facilitate cell growth and bone formation.
[0061] (2) Prepare 5% and 10% aqueous gelatin solutions, stir in a water bath at 50°C to promote dissolution, return to room temperature for use; prepare 5% glutaraldehyde aqueous solution.
[0062] (3) According to the dosage in Table 1, follow the steps below to prepare bone meal-gelatin films with different ratios.
[0063] Steps:
[0064] a. Quickly mix the ground bone meal powder with gelatin solution and glutaraldehyde solution on a glass slide, cover the surface of the mixed colloid with another glass slide, a...
Embodiment 2
[0074] (1) Grind the finished Bio-Oss bone powder (purchased from Geistlich Pharma AG) into a powder under sterile conditions, and the particle size of the ground powder particles is about 10um-200um observed under an environmental scanning electron microscope. The particle size is just suitable for mixing to make a scaffold material with uniform elasticity and hardness, so as to facilitate cell growth and bone formation.
[0075] (2) A gelatin aqueous solution with a concentration of 10% was prepared, stirred in a water bath at 50° C. to promote dissolution, and returned to room temperature for use; a 1% genipin aqueous solution stock solution was prepared (genipin was purchased from Shanghai Shifeng Biotechnology Co., Ltd.).
[0076](3) According to the dosage in Table 2, the gelatin / genipin-bone meal scaffold was prepared according to the following steps. The elastic modulus of the prepared gelatin / genipin-bone meal scaffold is shown in Table 2.
[0077] Steps:
[0078] a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pore size | aaaaa | aaaaa |
| Elastic modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com