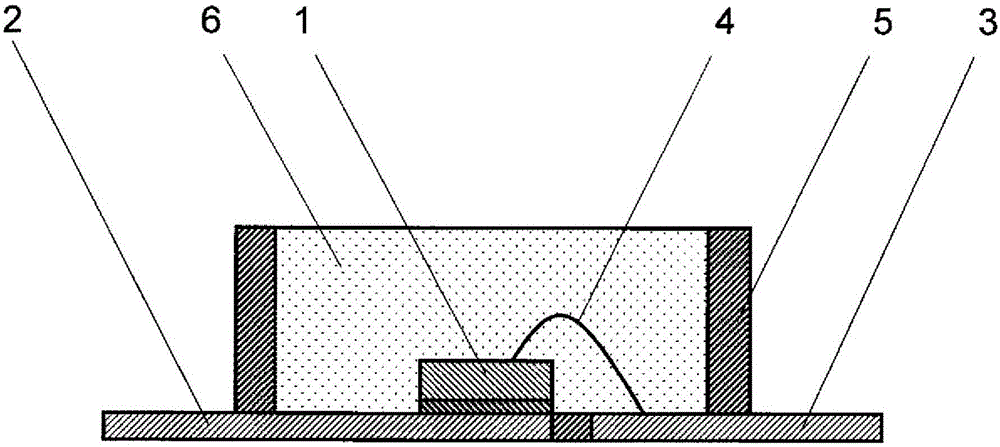
Curable silicone composition and optical semiconductor device
A composition and silicone technology, which is applied in semiconductor devices, semiconductor/solid-state device parts, electric solid-state devices, etc., can solve the problems of low light transmittance of cured products, insufficient adhesion of substrates, etc., and achieve excellent reliability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0106] In the above preparation method, it is characterized in that the silane compound (I-1) or the cyclic organosilicon compound (I-2), the silane compound (II) and the silane compound (III) are prepared in the presence of an acid or a base Hydrolysis condensation reaction.
[0107] Examples of the above-mentioned acid include hydrochloric acid, acetic acid, formic acid, nitric acid, oxalic acid, sulfuric acid, phosphoric acid, polyphosphoric acid, polycarboxylic acid, trifluoromethanesulfonic acid, and ion exchange resins. In addition, examples of the above-mentioned base include: inorganic bases such as potassium hydroxide and sodium hydroxide; triethylamine, diethylamine, monoethanolamine, diethanolamine, triethanolamine, ammonia water, tetramethylammonium hydroxide, Amino alkoxysilane, aminopropyltrimethoxysilane and other organic salt-based compounds.
[0108] In addition, in the above-mentioned production method, an organic solvent may be used. Usable organic solvent...
Embodiment
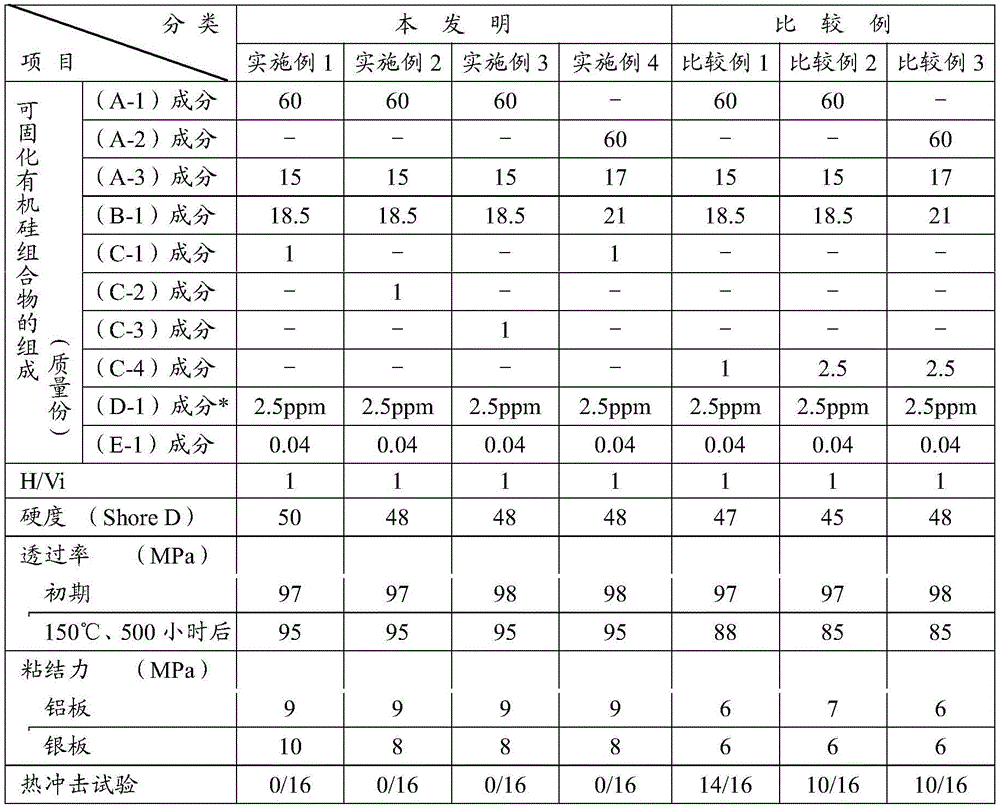
[0127] The curable silicone composition and optical semiconductor device of the present invention will be described in detail by way of examples. In addition, in the examples, the viscosity is a value at 25°C, and Me, Vi, Ph, and Ep represent a methyl group, a vinyl group, a phenyl group, and a 3-glycidoxypropyl group, respectively. In addition, the characteristics of the cured product of the curable silicone composition were measured as follows.
[0128] [Transmittance]
[0129] The curable silicone composition shown in Table 1 was poured into a mold having a length of 100 mm×a width of 10 mm×a thickness of 4 mm, and was cured by heating at 150° C. for 120 minutes. The transmittance at 450 nm was determined by putting the produced cured product test body into a quartz cell, and measuring the transmission spectrum with an automatic spectrophotometer.
[0130] [Adhesion]
[0131] [Adhesive force of cured product of curable silicone composition]
[0132] Between two aluminum...
reference example 1
[0138] Add 148.5 g (0.75 mol) of phenyltrimethoxysilane, 43.0 g (0.5 mol) of cyclic vinylmethylcyclosiloxane and trifluoromethanesulfonic acid into a reaction vessel with a stirrer, a reflux cooling pipe, and a thermometer 0.11g, add 21g of water. Then, it was made to reflux for 2 hours, and the low boiling point component was removed. Next, after cooling the reaction system, toluene and an aqueous potassium hydroxide solution were added. Thereafter, 88.1 g (0.4 mol) of 3-glycidoxypropylmethyldimethoxysilane and 14.4 g of water were added. After refluxing for 1 hour, methanol was distilled off, and excess water was removed by azeotropic dehydration. After heating under reflux for 5 hours, the toluene solution was cooled, neutralized with 1.1 g of acetic acid, and washed with water three times. After removing the water, toluene was distilled off under reduced pressure to prepare a product with a viscosity of 25.3 Pa·s, obtained by the average unit formula:
[0139] (PhSiO ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com