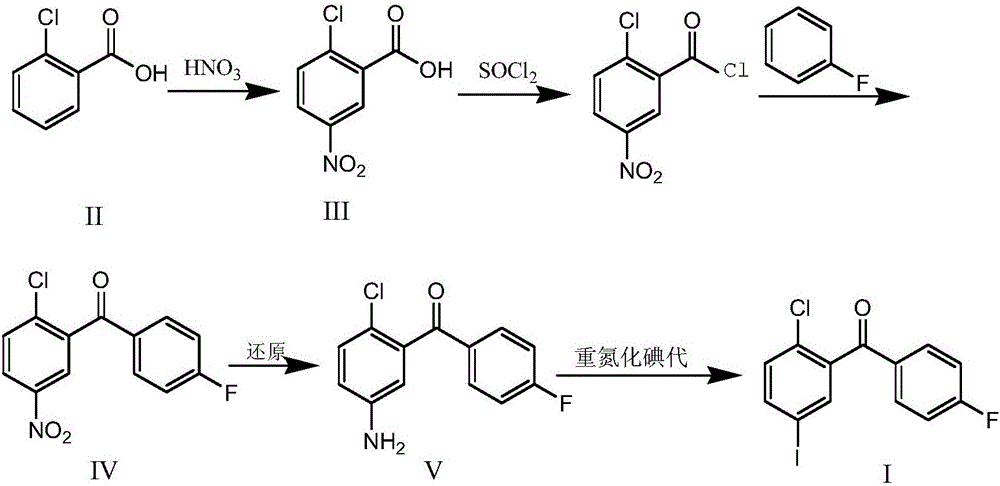
Synthesis method for (2-chloro-5-iodophenyl)(4-fluorophenyl)ketone
A synthetic method, the technology of fluorophenyl, applied in the field of drug synthesis, can solve the problems of not easy to obtain, high price, unsuitable for industrial production, etc., and achieve the effects of not easy to obtain, improve utilization rate, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Put 19.6kg of concentrated sulfuric acid into a 50L reaction kettle at room temperature, slowly add 3.12kg of o-chlorobenzoic acid, stir until it dissolves; cool down to -5~0°C, add 2.16kg of nitric acid (concentration 65%) dropwise, during the dropwise addition Temperature control -5-5°C; after dropping at 0-5°C for 2 hours, TLC (DCM:MeOH=10:1) to monitor the reaction, add 20kg of ice water dropwise to quench the reaction. Stir at a temperature below 25°C for 30 minutes, centrifuge, wash with 50 kg of water, and dry at 50°C under reduced pressure to obtain 3.83 kg of off-white solid 2-chloro-5-nitrobenzoic acid with a purity of 98.2% and a yield of 95.4%.
Embodiment 2
[0040] Put 2.1kg of 2-chloro-5-nitrobenzoic acid and 10kg of dichloromethane into a 30L reaction kettle at room temperature, add 100ml of anhydrous DMF, add 2.4kg of thionyl chloride dropwise, and control the temperature at 20~ 25°C, react under reflux for 3 hours after dropping, TLC (DCM:MeOH=10:1) monitors the completion of the reaction, then cool down to room temperature to obtain a dichloromethane solution of 2-chloro-5-nitrobenzoyl chloride for later use;
[0041]Add 10kg of dichloromethane and 2.3kg of aluminum trichloride into a 50L reaction kettle, cool down to 0°C, stir and add 1.5kg of fluorobenzene, then keep the above 2-chloro-5-nitrobenzoyl chloride at 5°C The dichloromethane solution was added dropwise into the reaction kettle, and the temperature of the reaction solution was controlled at 0-5° C. and stirred for 5 hours. After TLC (PE / EA: 1 / 3) monitoring reaction finishes, reaction solution is transferred in the 100L reactor that 30L ice water is housed, stirred...
Embodiment 3
[0043] Add 10.4kg of (2-chloro-5-nitrophenyl)(4-fluorophenyl)methanone, 5.9kg of iron powder, 80kg of ethanol, 20kg of water and 11.5kg of ammonium chloride into a 200L reactor, and heat to 78 Reflux reaction at ~80°C for 5h, TLC (PE / EA:1 / 3) to monitor the end of the reaction, filter while hot, wash with 10kg of hot ethanol, evaporate the filtrate to dryness at 60°C to obtain the crude product. Then add 65kg of ethyl acetate, heat and reflux for beating for 2h, drop to 0-5°C for crystallization for more than 30min, centrifuge, wash with 3kg of glacial ethyl acetate, dry under reduced pressure at 50°C to obtain a yellow solid (2-chloro-5-aminophenyl) (4-fluorophenyl)methanone 8.60kg, purity 99.1%, yield 92.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com