Solid lipid magnetic resonance nanoparticle as well as preparation method and application thereof
A nanoparticle and solid lipid technology, applied in emulsion delivery, drug delivery, etc., can solve problems such as weak affinity, achieve low toxicity, high cell survival rate, and solve the problem of burst release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Preparation of solid lipid nanoparticles loaded with Gd-DTPA
[0032] (1) First weigh 54mg Tween-80 and dissolve it in 3ml water to form the water phase, and dissolve 600mg Span-80 into 30ml n-hexane to form the organic phase, and add the water phase to the organic phase under stirring at room temperature at 400rpm, Probe ultrasound to make submicron emulsion;
[0033] (2) Then weigh 62.6mg of gadopentetate meglumine and dissolve in water to obtain an aqueous solution, dissolve 18mg of octadecylamine in 3ml of ethanol to obtain an ethanol solution, then combine the two at 60°C for 30min, and spin in vacuum at 60°C The solvent is evaporated to obtain an intermediate product;
[0034] (3) Add 3ml of ethanol, 90mg of monoglyceride and 10mg of lecithin mixture to the intermediate product prepared in step (2), inject it into the submicron emulsion prepared in step (1) under heating condition at 60°C, stir at room temperature for 5min; then centrifuge at 20000r...
Embodiment 2
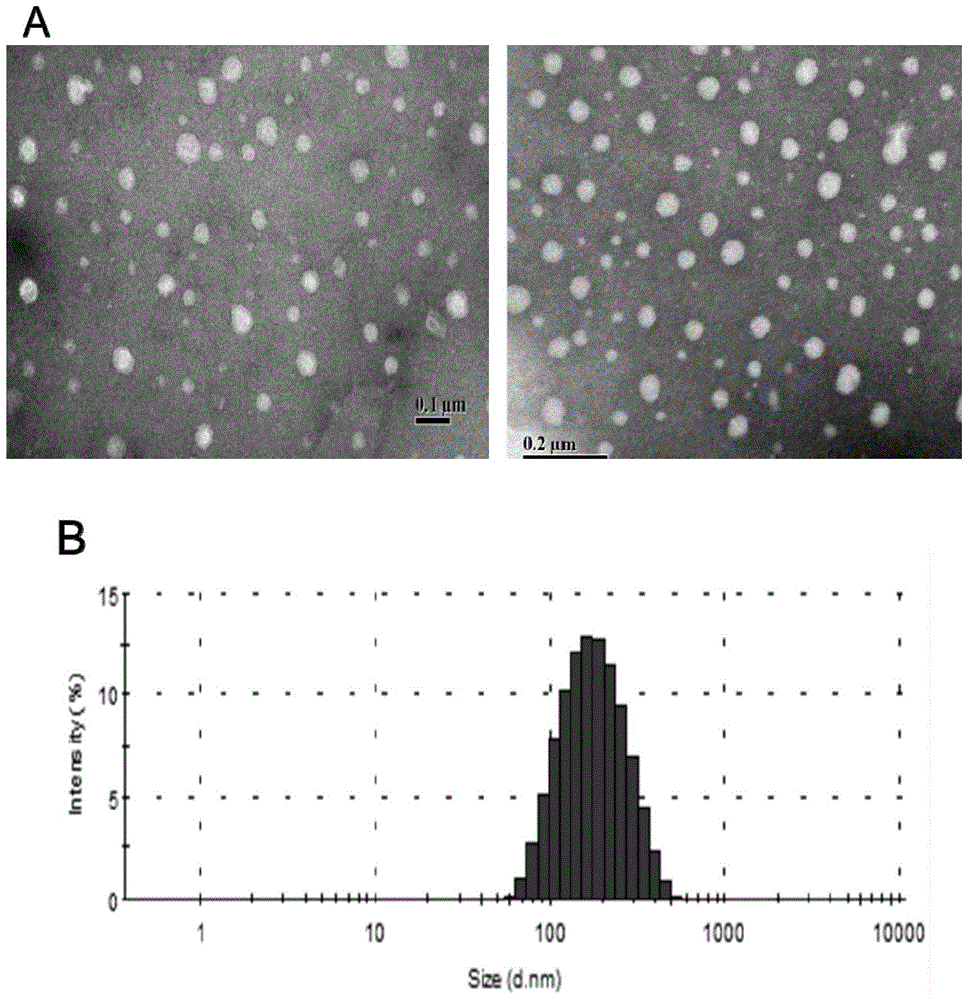
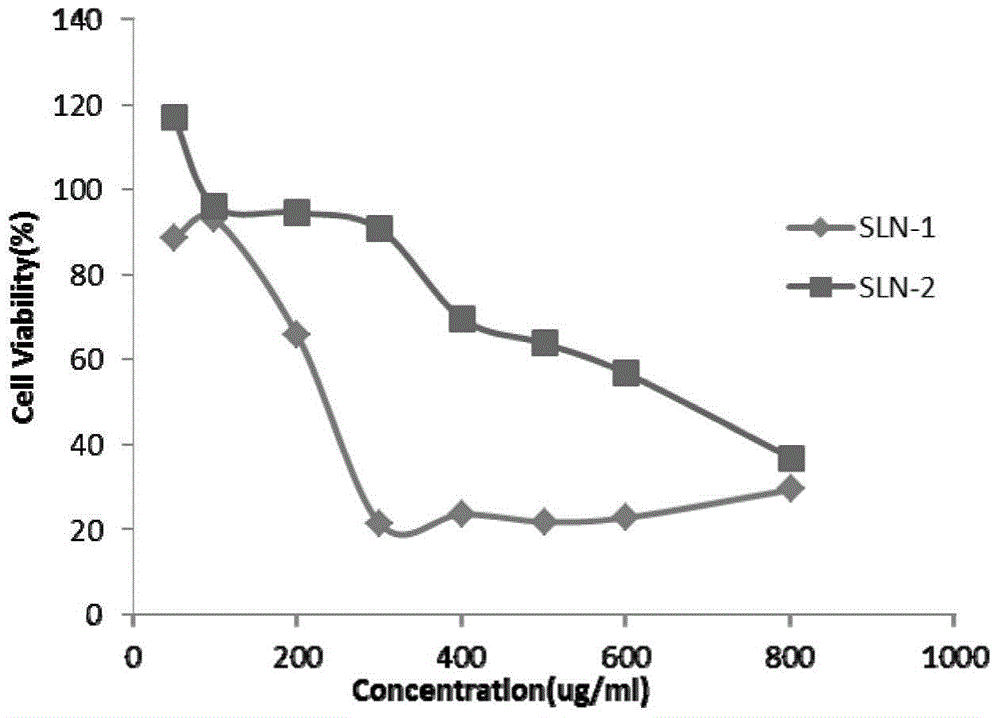
[0035] Embodiment 2: The physicochemical property investigation of the solid lipid nanoparticle of loading Gd-DTPA
[0036] Take the solid lipid nanoparticles prepared above, use ultrapure water as a reconstitution solvent, and use a 3000HS particle size and surface potential analyzer to measure its particle size at a concentration of 0.01 mg / ml.
[0037] The encapsulation efficiency of Gd-DTPA in solid lipid nanoparticles was determined by indirect method. Fluorescence spectrophotometry (Ex=495nm, Em=514nm, Slit=5nm) measures the fluorescence value, calculates the amount of free Gd-DTPA in the solution, and calculates the encapsulation efficiency of the fluorescent graft by (1) formula:
[0038] Gd-DTPA encapsulation efficiency = (Wo-W free) / Wo*100% (1)
[0039]Gd-DTPA drug loading is calculated according to formula (2):
[0040] Gd-DTPA drug loading = (added drug * encapsulation efficiency) / (added drug * encapsulation efficiency + carrier material amount) * 100%
[0041...
Embodiment 3
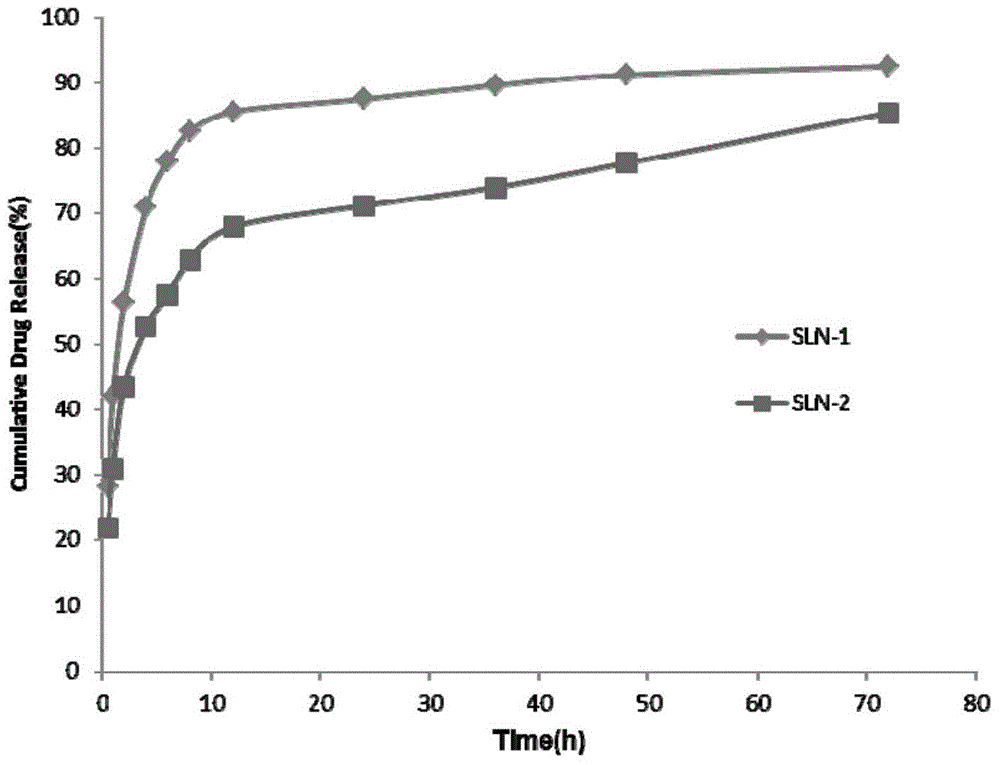
[0043] Example 3: Research on in vitro drug release behavior of solid lipid nanoparticles loaded with Gd-DTPA
[0044] A certain volume of SLN solution was pipetted, placed in a dialysis bag (MWCO 3.5KDa) and then placed into a release tube containing 25ml of release medium (pH 7.2PBS). In vitro release was carried out at 37°C and 65rpm constant temperature shaking, samples were taken at specific time points (0.5h, 1h, 2h, 4h, 6h, 8h, 12h, 24h, 36h, 48h and 72h), and all release media were replaced at the same time. Measure the drug concentration in the sample by fluorescence spectrophotometry (Ex=275nm, Em=313nm, slit=5nm, working voltage=700V), and calculate the cumulative release and cumulative release percentage of the drug. The result is as image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com