Liver clearing and toxin removing tablets and preparation method
A technology for clearing liver and detoxification, and Schisandra chinensis, which is applied in the directions of antiviral agents, pharmaceutical formulations, medical preparations with inactive ingredients, etc., can solve the problems of low bioavailability, long disintegration time, low dissolution rate, etc. Simple, improved dissolution and bioavailability, low cost of production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] A preparation method of Qingganjiedu tablet, comprising the following steps:
[0077] (1) Take each raw material according to the following parts by weight: 300 parts of Schisandra chinensis, 300 parts of Polygonum cuspidatum, 300 parts of Yexiazhu, 300 parts of Gynostemma pentaphyllum, 300 parts of Salvia miltiorrhiza, 200 parts of Ganoderma lucidum, 200 parts of charcoal mother, 200 parts of rhubarb, Bupleurum 200 parts, Liushenqu 200 parts, licorice root 200 parts, starch 500 parts, sucrose 150 parts, β-cyclodextrin 30 parts, sucrose fatty acid ester 90 parts, Cordyceps sinensis 150 parts, gelatin syrup 80 parts, simethicone 5 parts 200 parts magnesium stearate and 5000 parts ethanol.
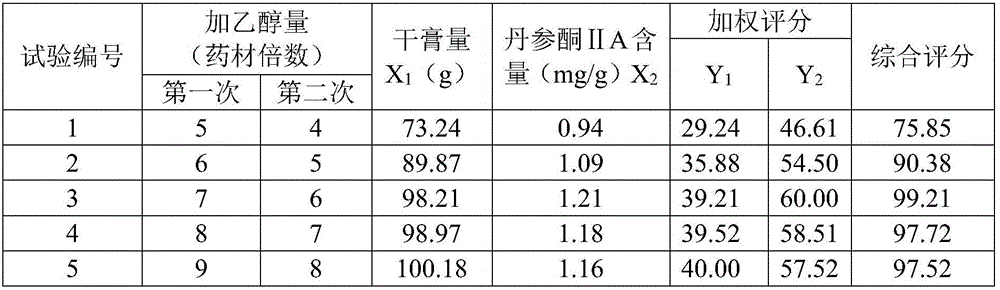
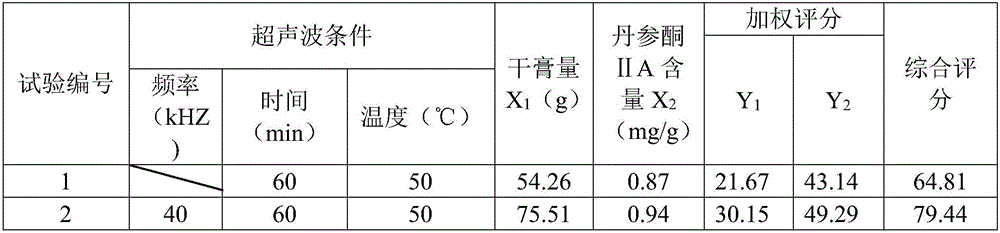
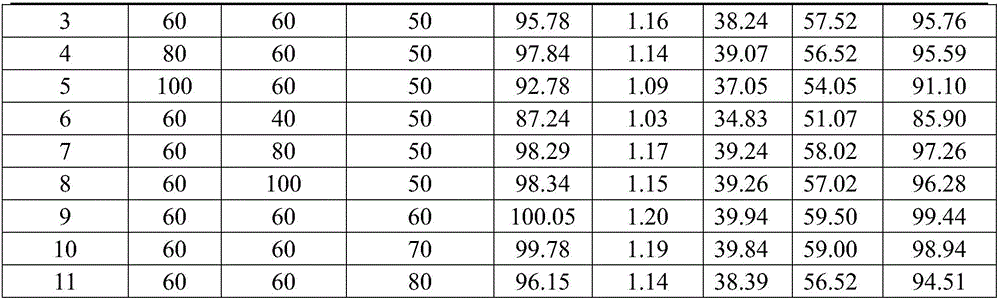
[0078] (2) get Schisandra chinensis, Salvia miltiorrhiza and Bupleurum chinensis ethanol ultrasonic extraction 2 times, the add-on of ethanol for the first time is 7 times the weight of the medicinal material, the add-on for the second time is 6 times the weight of ethanol, and the ul...
Embodiment 2
[0083] A preparation method of Qingganjiedu tablet, comprising the following steps:
[0084] (1) Take each raw material according to the following parts by weight: 300 parts of Schisandra chinensis, 300 parts of Polygonum cuspidatum, 300 parts of Yexiazhu, 300 parts of Gynostemma pentaphyllum, 300 parts of Salvia miltiorrhiza, 200 parts of Ganoderma lucidum, 200 parts of charcoal mother, 200 parts of rhubarb, Bupleurum 200 parts, 200 parts Liushenqu, 200 parts licorice, 600 parts starch, 100 parts sucrose, 20 parts β-cyclodextrin, 100 parts sucrose fatty acid ester, 100 parts Cordyceps sinensis, 50 parts gelatin syrup, 10 parts simethicone 300 parts magnesium stearate and 6000 parts ethanol.
[0085] (2) get Schisandra chinensis, Salvia miltiorrhiza and Bupleurum chinensis ethanol ultrasonic extraction 2 times, the add-on of ethanol for the first time is 7 times the weight of the medicinal material, the add-on for the second time is 6 times the weight of ethanol, and the ultra...
Embodiment 3
[0090] A preparation method of Qingganjiedu tablet, comprising the following steps:
[0091] (1) Take each raw material according to the following parts by weight: 300 parts of Schisandra chinensis, 300 parts of Polygonum cuspidatum, 300 parts of Yexiazhu, 300 parts of Gynostemma pentaphyllum, 300 parts of Salvia miltiorrhiza, 200 parts of Ganoderma lucidum, 200 parts of charcoal mother, 200 parts of rhubarb, Bupleurum 200 parts, Liushenqu 200 parts, licorice 200 parts, starch 550 parts, sucrose 200 parts, β-cyclodextrin 40 parts, sucrose fatty acid ester 90 parts, Cordyceps sinensis 200 parts, gelatin syrup 100 parts, simethicone 8 parts parts, magnesium stearate 250 parts and ethanol 550 parts.
[0092] (2) get Schisandra chinensis, Salvia miltiorrhiza and Bupleurum chinensis ethanol ultrasonic extraction 2 times, the add-on of ethanol for the first time is 7 times the weight of the medicinal material, the add-on for the second time is 6 times the weight of ethanol, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com