Colloidal gold test strip capable of simultaneously detecting waterfowl source parvoviruses and preparation method
A technology of colloidal gold test paper and parvovirus, applied in the field of veterinary medicine, can solve the problems of limited application and narrow use range, and achieve the effects of reducing false positives, high sensitivity, and improving specificity and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Preparation of Gosling Plague Virus Monoclonal Antibody
[0027] 1.1 Immunization of mice
[0028] Concentrated and purified gosling plague virus was used as antigen, emulsified with Freund's complete adjuvant, injected subcutaneously into mice at multiple points, and boosted with the same antigen plus Freund's incomplete adjuvant for 5 times every 2 weeks. One week after the last immunization, blood was collected to prepare serum, and the titer was determined by indirect ELISA method. Mice whose serum titer reached 1:1000 and above were given a booster immunization without adjuvant antigen, and cell fusion was carried out 3 days later.
[0029] 1.2 Preparation of myeloma cells
[0030] 48 hours before fusion, SP2 / 0 myeloma cells were expanded and cultured in cell culture dishes with a diameter of 9 cm, and 10 mL of DMEM medium (product of Gibco Company) containing 10% fetal bovine serum was added to each culture dish, and placed at 37°C, 5%CO 2 Culture in...
Embodiment 2
[0053] Example 2: Colloidal gold labeling of monoclonal antibody 3D9
[0054] 2.1 Preparation of colloidal gold
[0055] Take 1mL of 10g / L chloroauric acid solution and add it to the Erlenmeyer flask that has been treated with soaking acid and siliconization, add 100mL deionized water, seal it with tin foil, and put it in the microwave oven. Bring the solution to boil on high heat for 3 minutes, take out the Erlenmeyer flask, quickly add 1.5mL of 10g / L trisodium citrate solution, and mix well at the same time. The solution will change from pale yellow to colorless to grayish black. Seal with tin foil, put the Erlenmeyer flask into the microwave oven, heat it on medium-high heat for 3 minutes, after the solution turns wine red, heat it for another 3 minutes, after it cools down to room temperature, fill it up to 100mL with deionized water, and filter it with a 450nm filter membrane , stored at 4°C protected from light.
[0056] 2.2 Colloidal gold labeling
[0057] Centrifug...
Embodiment 3
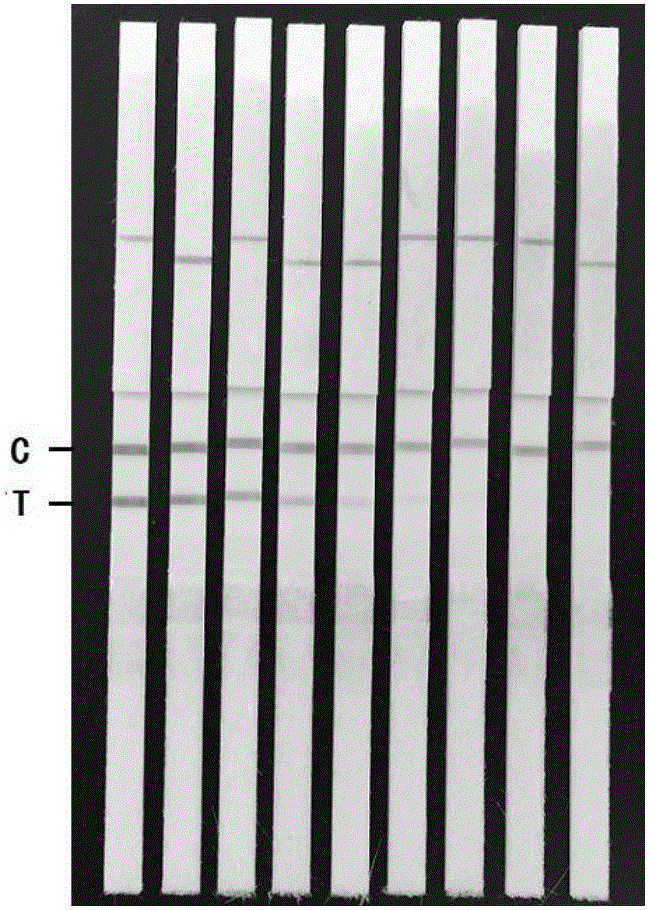
[0058] Embodiment 3: the assembly of colloidal gold test strip
[0059] 3.1 Preparation of gold standard pad
[0060] Soak the glass cellulose membrane in the colloidal gold-labeled monoclonal antibody 3D9, let it stand at 37°C for 30 minutes, and dry it at room temperature to form a gold label pad, which is stored at 4°C.
[0061] 3.2 Preparation of nitrocellulose membrane (NC membrane)
[0062] Spray 2 mg / mL of monoclonal antibody 5F4 on the test line (T) on the NC membrane with a membrane spraying system; spray 1 mg / mL of goat anti-mouse IgG antibody on the quality control line (C) on the NC membrane with a membrane spraying system ). Dry at room temperature, soak in 20g / L BSA solution, let stand at room temperature for 30min, wash with PBST 3 times, 5min each time, dry at room temperature, that is, NC membrane, and store at 4°C.
[0063] 3.3 Assembly
[0064] Paste the sample pad, the prepared gold standard pad, NC film and absorbent paper on the plastic bottom plate i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com