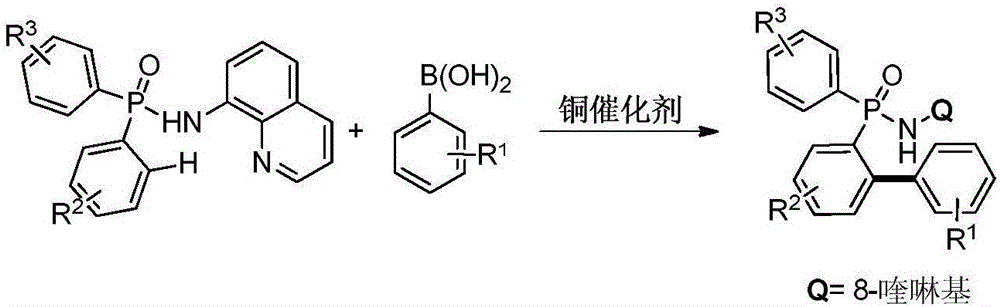
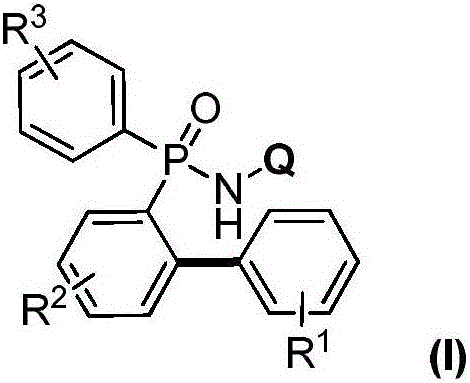
Preparation method for aromatic phosphine compound
A technology for aromatic phosphine compounds, which is applied in the field of preparation of aromatic phosphine compounds, and achieves the effects of mild reaction conditions, good industrial application prospects, superior conversion rate and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0027] Add 0.005mmol copper powder, 0.1mmol P,P-diphenyl-N-(8-aminoquinoline)-phosphonamide, 0.2mmol phenylboronic acid in turn to the Schlenk reaction tube, vacuum backfill oxygen; dropwise under oxygen atmosphere 1mL of acetonitrile was used as a solvent, and the reaction was continued at 100°C for 6h; after the reaction was completed, it was cooled to room temperature and separated by column chromatography to obtain the target product: P-(2-phenylphenyl)-P-phenyl-N-(8 -aminoquinoline)-phosphonamide in 93% yield.
preparation example 2
[0029] Add 0.005mmol cuprous oxide, 0.1mmol P,P-diphenyl-N-(8-aminoquinoline)-phosphonamide, and 0.2mmol phenylboronic acid in turn to the Schlenk reaction tube, vacuum backfill oxygen; drop under oxygen atmosphere Add 1mL of acetonitrile as a solvent, and continue to react at 100°C for 6h; after the reaction, cool to room temperature and separate by column chromatography to obtain the target product: P-(2-phenylphenyl)-P-phenyl-N-( 8-aminoquinoline)-phosphonamide in 91% yield.
preparation example 3
[0031] Add 0.005mmol copper chloride, 0.1mmol P,P-diphenyl-N-(8-aminoquinoline)-phosphonamide, 0.1mmol phenylboronic acid in turn to the Schlenk reaction tube, vacuum backfill oxygen; drop under oxygen atmosphere Add 1mL of acetonitrile as a solvent, and continue to react at 100°C for 6h; after the reaction, cool to room temperature and separate by column chromatography to obtain the target product: P-(2-phenylphenyl)-P-phenyl-N-( 8-aminoquinoline)-phosphonamide in 92% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com