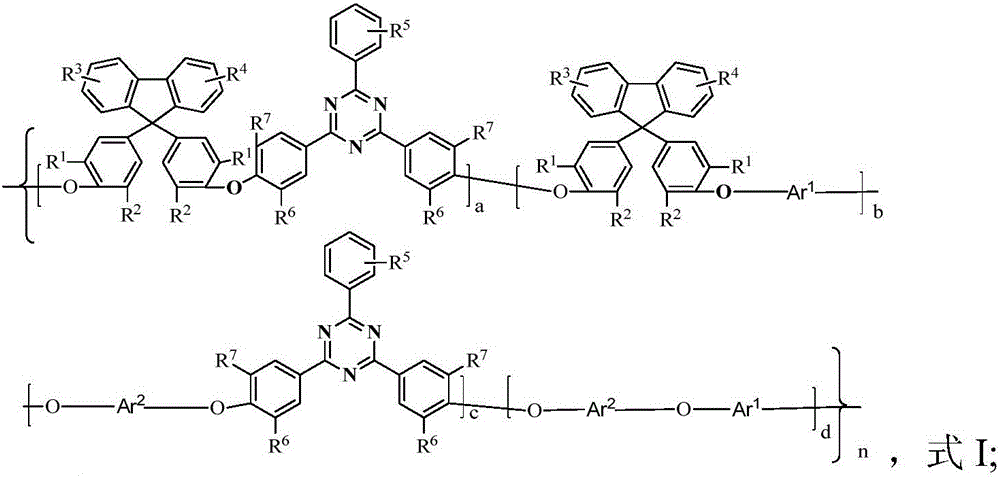
Polyarylether with main chain containing bisbenzofiurene and triaryl-s-triazine structure and preparation method of polyarylether
A technology of bisphenyl fluorene and s-triazine, which is applied in the field of polymer material synthesis, can solve problems such as research on synthesis of polyaryl ethers that are not involved, and achieve the effects of improved solubility and excellent solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] When the polyarylether is prepared by a bisphenol monomer containing bisphenylfluorene and a dihalogen monomer containing triaryl-s-triazine rings, the preparation method includes the following steps in sequence:
[0048] Add the bisphenol monomer containing bisphenylfluorene, alkali catalyst, solvent and dehydrating agent to the reaction kettle with water separator, stirrer, thermometer and nitrogen inlet pipe in sequence, and first raise the temperature to 120℃~150℃ Dehydration for 1-7 hours, distill out the dehydrating agent, cool to room temperature, add equimolar dihalogen monomer containing triaryl-s-triazine ring; then heat up to 100-220°C and react for 1-30 hours, then add precipitant, The polymer is coagulated and precipitated; the copolymer is obtained through suction filtration, separation, washing, drying and other steps; the molar yield is 90-99%.
[0049] The polyaryl ether can also be passed through bisphenol monomers containing bisphenylfluorene, bisphen...
Embodiment 1
[0067] (1) Add 0.1101g of hydroquinone (HQ) and 1.4000g of 9,9-bis(4-hydroxyphenyl)fluorene to a 100mL three-necked flask equipped with mechanical stirring, water separator condenser and nitrogen inlet (BHF), 0.8293g of anhydrous potassium carbonate, 5mL of sulfolane and 20mL of toluene, refluxed at 150°C with water for 3h, distilled off the toluene, and cooled to room temperature.
[0068] (2) Add 1.7267g of 2,4-bis(4-fluorophenyl)-6-phenyl-1,3,5-triazine (BFPT) into the three-necked flask again, and pass N 2 The incoming air was excluded for 20 minutes, the temperature was raised to 190° C. for 7 hours, and the solvent sulfolane was continuously added to obtain the diluted product.
[0069] (3) sink the product after the sulfolane dilution into boiling water containing a small amount of hydrochloric acid and keep stirring to obtain a white strip polymer, the crude product (detected by GPC, which contains about 3% of small molecules, so it is necessary for further purificat...
Embodiment 2
[0078] (1) Add 6.72g of 2,2-bis-(4-hydroxyphenyl)hexafluoropropane (BAF), 7.00g of 9 , 9-bis(4-hydroxyphenyl)fluorene (BHF), 6.40g of anhydrous potassium carbonate, 10mL of N,N-dimethylacetamide and 50mL of toluene, refluxed at 150°C with water for 3h, distilled off the toluene, Cool to room temperature.
[0079] (2) Add 13.80 g of 2,4-bis(4-fluorophenyl)-6-phenyl-1,3,5-triazine (BFPT) into the three-necked flask again, and pass N 2 The air entering was removed for 20 minutes, the temperature was raised to 160°C for 7 hours, and the solvent N,N-dimethylacetamide was continuously added.
[0080] (3) After the reaction is completed, the product diluted with N,N-dimethylacetamide is immersed in boiling water containing a small amount of hydrochloric acid and stirred continuously to obtain a white strip polymer, that is, the crude product.
[0081] (4) Boil the crude product in distilled water until it boils, and keep it for 2 hours, then use a Buchner funnel to suction filter,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| heat deflection temperature | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com