Protein with chemical modified groups and preparation method thereof
A chemical modification and protein technology, applied in the field of protein drug modification, can solve problems such as non-specific modification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1: Preparation of protein C
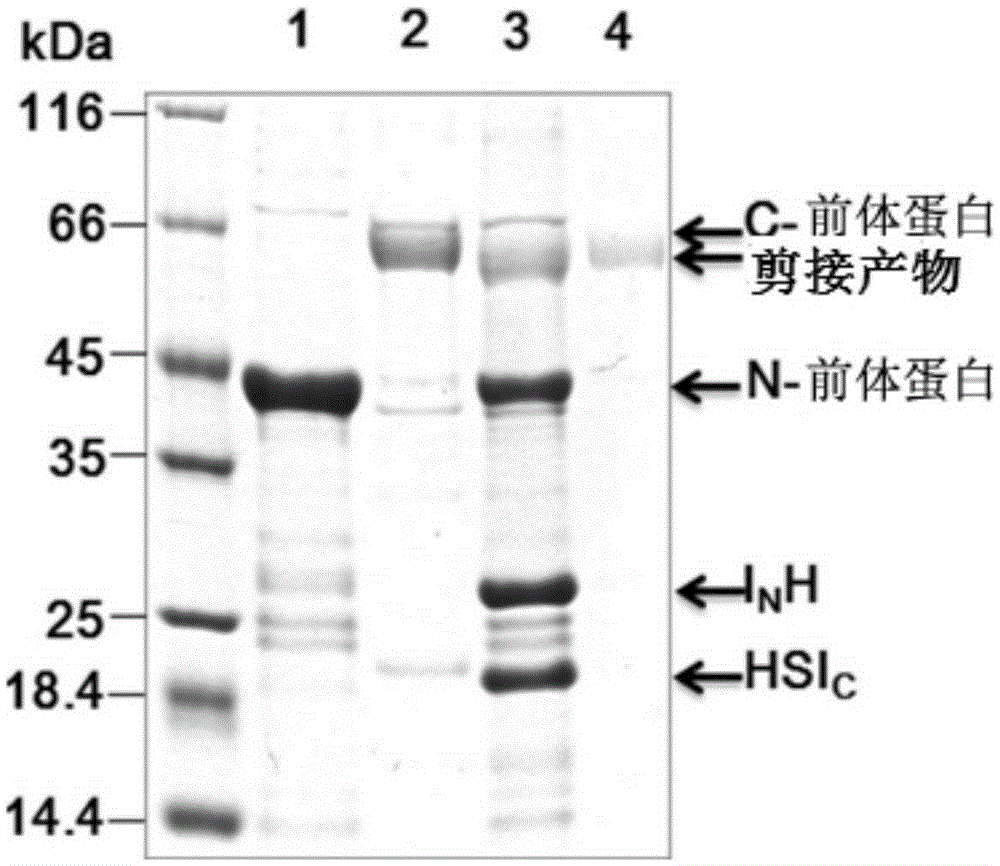
[0060] We selected the genetically engineered Ssp GyrB break intein for constructing the C-protein. C-protein belongs to the fusion protein, which in this embodiment contains 6×histidine tag, SUMO protein (SUMO, Small Ubiquitin-like Modifier protein), SG (Ssp GyrB) intein C segment (SGsplit I C , SEQ ID NO:1), and the amino acid sequence SAGC containing only one cysteine (ie the first amino acid sequence, SEQ ID NO:3). In addition, another option for the C-protein is to sequentially contain a 6×histidine tag, thioredoxin (T protein), the C segment of the SG (Ssp GyrB) intein (SGsplit I C , SEQ ID NO:1), and the amino acid sequence SAGC containing only one cysteine (ie the first amino acid sequence, SEQ ID NO:3). Of course, those skilled in the art can understand that other tag proteins include maltose binding protein (MBP protein) and glutathione sulfhydryl transferase (GST).
[0061] Specifically, a 6×histidine tag was us...
Embodiment 2
[0063] Embodiment 2: Preparation and preparation of N-protein
[0064] In order to verify the generality of the method, a variety of protein peptide drugs were regarded as target proteins. These protein peptide drugs include granulocyte colony stimulating factor (Granulocyte Colony-Stimulating Factor, G-CSF), human growth hormone (human Growth Hormone, hGH), human interferon α2b (interferon-α2b, IFNα2b), interleukin-15 (Interleukin 15, ILK-15) and uric acid oxidase (Urate Oxidase, UOX). Various above-mentioned protein polypeptide drugs (ie the second amino acid sequence, the specific sequence can be seen in SEQ ID NO: 6-10 in the sequence listing) are sequentially combined with the N fragment of the intein (SGsplitI N ) (SEQ ID NO:2) and 6×His tag fusion expression, that is, N protein (N drug I N h).
[0065] The nucleotide sequence of each N-protein was cloned into a PET-28 vector, pE-N drug I N H. According to the standard conversion method, the various N drug I N H...
Embodiment 3
[0066] Example 3: PEGylation and purification of C-protein
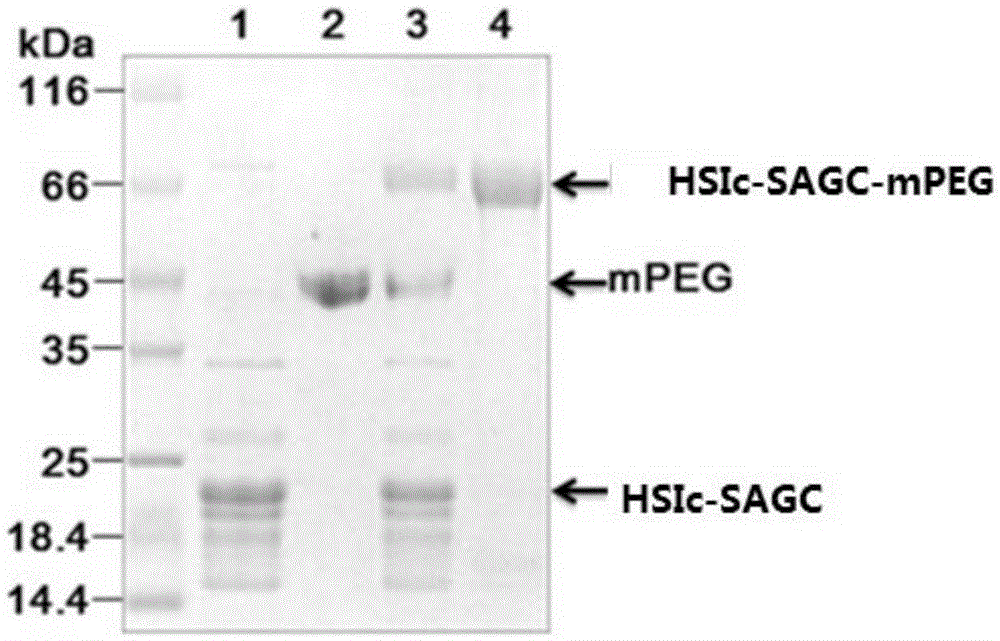
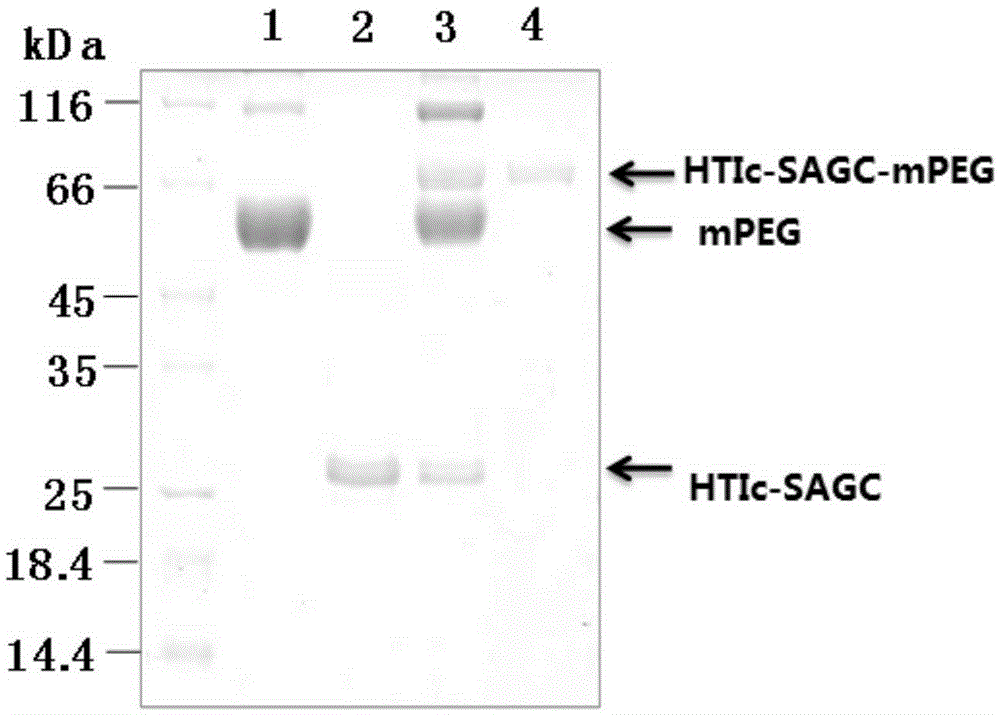
[0067] The schematic diagram of PEGylation and purification of C-protein is shown in Fig. 1 . First the C-protein (HS (or T) I obtained in Example 1 C -SAGC) dialyzed into labeling buffer (140mM NaCl, 2.7mM KCl, 10mM NaCl 2 HPO 4 , 1.8mM KH 2 PO 4 , pH 7.3), and added tris (2-carboxyethyl) phosphine (TCEP) to a final concentration of 1-10 mM. Add thiol-active polyethylene glycol (PG1-ML-20k, NANOCS), the molar ratio of polyethylene glycol to protein is 3-10:1, and place at room temperature for 2.5-12 hours. After the labeling reaction was completed, dithiothreitol (DTT) was added to a final concentration of 1-10 mM. Due to the C-protein (HS(or T)I C -SAGC) there is only one cysteine, so the PEG with sulfhydryl reactive functional group is modified to C-protein (HS(or T)I C -SAGC) at specific sites that form the C-precursor protein (HS(or T)I C -SAGC-mPEG). Use SDS-PAGE gel to detect polyethylene glycol modi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com