Oral insulin composition
An insulin and composition technology, applied in the field of oral insulin composition, can solve the problems of unsolvable stability and low bioavailability of oral insulin, and achieve the effect of good hypoglycemic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: the preparation of insulin composition
[0050] 1) Dissolve 1 g of insulin in 10 ml of hydrochloric acid solution (pH 1-2), and adjust the pH to 4 with sodium hydroxide solution (10%-20%) after dissolving;
[0051] 2) Add 3g of gelatin to 150ml of distilled water to dissolve into a gelatin solution;
[0052] 3) adding the insulin solution into the gelatin solution under stirring, mixing evenly, adjusting the pH to 5-8 with 10% NaOH solution, the complex is precipitated, and an insulin-gelatin complex suspension is formed;
[0053] 4) 0.02g of glycocholic acid, 25g of sodium taurocholate, 145g of lecithin, 0.7g of cholesterol, 0.2g of bilirubin and 0.05g of chloroquine were dissolved in absolute ethanol to form a solution, and the ethanol was recovered under reduced pressure, and dried to prepare mixture;
[0054] 5) adding the above mixed solution to the insulin-gelatin complex suspension to form insulin complex liposomes;
[0055] 6) Add 25 g of microc...
Embodiment 2
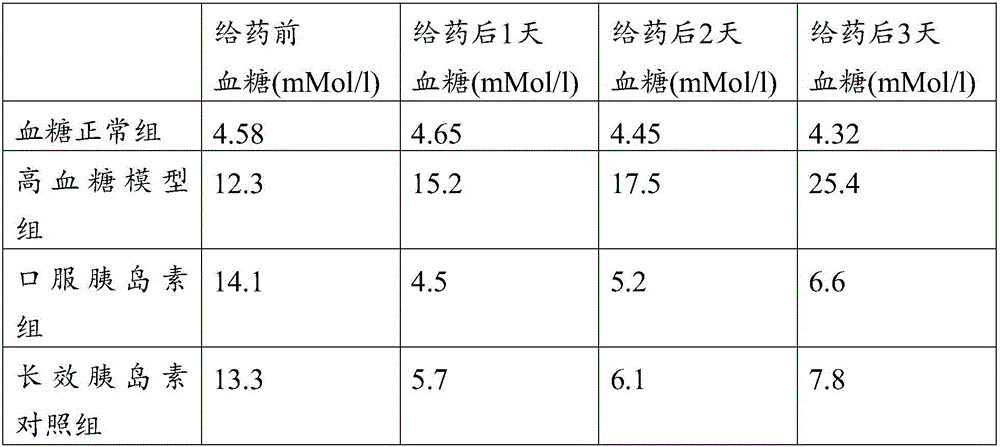
[0057] Example 2: In vivo effect comparison between insulin composition and commercially available long-acting insulin
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com