Electrochemical synthesis method of 1,1'-diindolylmethane derivatives
A technology of diindolylmethane and indole derivatives, applied in electrolysis process, electrolysis components, electrolysis organic production and other directions, can solve the problem of high cost and achieve the effects of low cost, wide application range and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
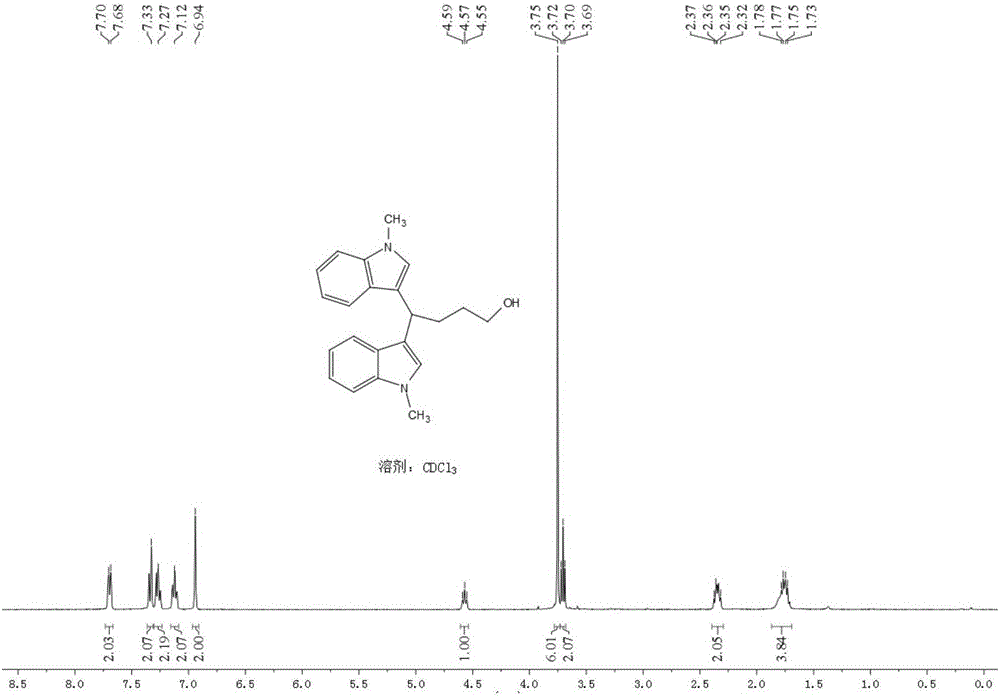
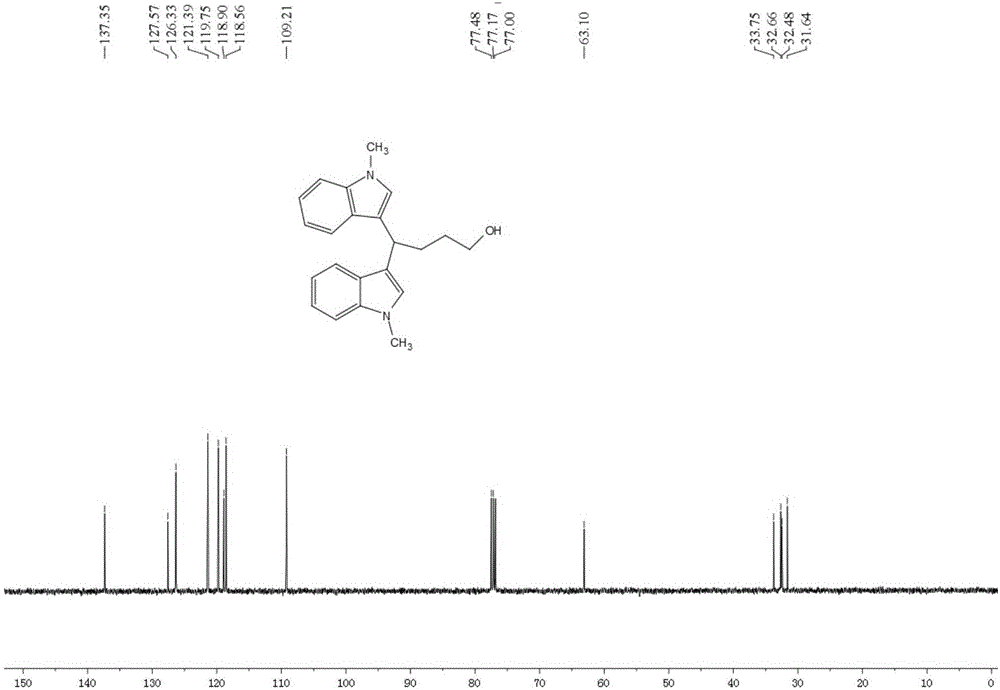
[0045] Add 19.5mg (0.08mmol) LaCl to a 5ml round-bottomed flask 3 ,106.4mg(0.2mmol / ml)LiCIO 4 , 104.9 mg (0.8 mmol) N-methyl indole; then 3.3 ml of tetrahydrofuran and 1.7 ml of acetonitrile were added. Insert two electrodes (the platinum wire is the anode and the platinum sheet is the cathode), the DC power supply is powered at 5mA, the reaction is stirred and monitored by TLC, and the reaction is complete in 4.5h. The crude product was extracted with ethyl acetate (15ml×3), the organic layers were combined, washed with saturated aqueous NaCI solution (40ml×1), and anhydrous Na 2 SO 4 After drying, evaporated to dryness under reduced pressure, the product 1 was isolated in a yield of 82.4%.
[0046]
Embodiment 2
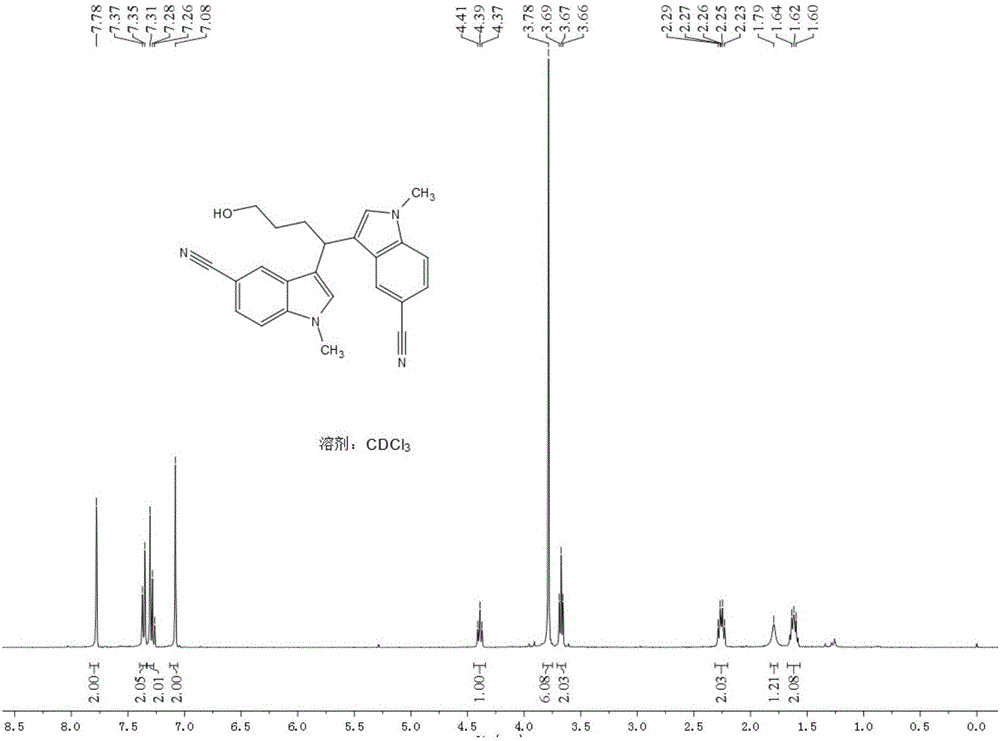
[0048] Add 19.5mg (0.08mmol) LaCl to a 5ml round-bottomed flask 3 , 106.4mg (the concentration in the electrolytic solvent is 0.2mmol / ml) LiCIO 4 , 104.9 mg (0.8 mmol) N-methyl indole; then 3.3 ml of tetrahydrofuran and 1.7 ml of acetonitrile were added. Insert two electrodes (the platinum wire is the anode and the platinum sheet is the cathode), the DC power supply is powered at 2.5mA or 8mA, the reaction is stirred and monitored by TLC, and the reaction is complete in 4.5h. The crude product was extracted with ethyl acetate (15ml×3), the organic layers were combined, washed with saturated aqueous NaCI solution (40ml×1), and anhydrous Na 2 SO 4 Dry, evaporate to dryness under reduced pressure, and isolate the product 1 in a yield of 32.8% (2.5mA) or 45.7% (8mA).
[0049]
Embodiment 3
[0051] Add 19.5mg (0.08mmol) LaCl to a 5ml round-bottomed flask 3 , 106.4mg (the concentration in the electrolytic solvent is 0.2mmol / ml) LiCIO 4 , 104.9 mg (0.8 mmol) of N-methyl indole; then 5 ml of a mixed solvent of tetrahydrofuran / acetonitrile (v / v) (1:1 or 3:1) was added. Insert two electrodes (the platinum wire is the anode and the platinum sheet is the cathode), the DC power supply is powered at 5mA, the reaction is stirred and monitored by TLC, and the reaction is complete in 4.5h. The crude product was extracted with ethyl acetate (15ml×3), the organic layers were combined, washed with saturated aqueous NaCI solution (40ml×1), and anhydrous Na 2 SO 4 Dry and evaporate to dryness under reduced pressure to obtain product 1 in a yield of 47.5% (1:1) or 62.3% (3:1).
[0052]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com