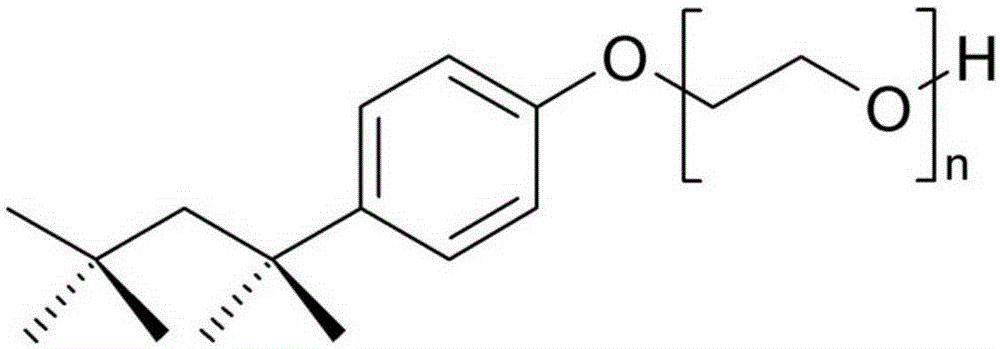
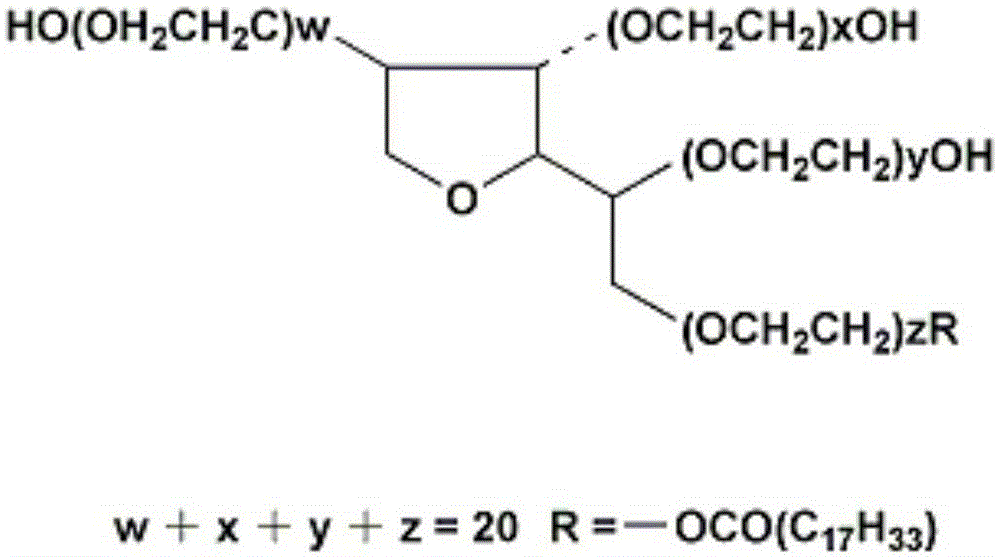
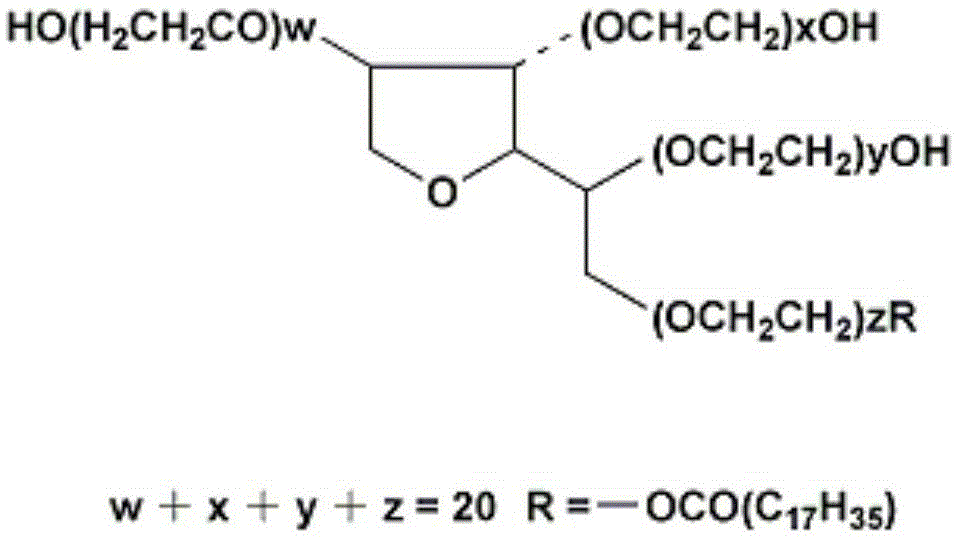
Human immunodeficiency virus antigen and antibody combined detection kit, use and detection method thereof
A technology of human immunodeficiency and detection kit, which is applied in the field of human immunodeficiency virus detection to achieve the effects of reliable detection results, improved early detection rate and high detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0029] According to one embodiment of the present invention, the detection kit comprises the following components:
[0030] (a) Magnetic particle coating: HIV-1 / M specific antigen, HIV-1 / O specific antigen, HIV-2gp36 antigen and HIV p24 antibody are coated on the magnetic particle, present in the magnetic particle diluent ;
[0031] (b) Enzyme markers: labeled enzyme-labeled HIV-1 / M-specific antigens, HIV-1 / O-specific antigens, HIV-2gp36 antigens and HIV p24 antibodies, present in enzyme marker diluents;
[0032] (c) Sample Treatment Fluid: Contains a non-ionic surfactant; and
[0033] (d) Test diluent: buffer used to dilute the sample to be tested.
[0034] The above four components are described in detail below. It should be noted that, unless otherwise specified, the content percentage (%) in the present invention refers to weight volume percentage (W / V%), for example, 1.0% refers to 1.0W / V%, wherein weight ( The units of W) and volume (V) may be grams (g) and millilite...
Embodiment 1
[0079] The HIV antigen and antibody combined detection kit of this embodiment contains the following components: magnetic particle coating working solution (Ra), enzyme marker working solution (Rb), sample processing solution (Rc) and test diluent (Rd). The process of kit preparation is as follows:
[0080] 1. Preparation of magnetic particle coating
[0081] The process of linking specific antibodies or antigens to magnetic beads by covalent coupling is called magnetic particle coating; the prepared magnetic particle and antibody or antigen conjugate is called magnetic particle coating.
[0082] In the preparation of the HIV kit in this example, the antigens or antibodies used to coat the magnetic particles include HIV-1 / M specific gp41 antigen, HIV-1 / O specific gp41 antigen, HIV-2 gp36 antigen and HIV p24 antibody IV Among them, the HIV p24 antibody is a monoclonal antibody of two sources (respectively, a mouse-derived antibody and a human-derived antibody). The basic prin...
Embodiment 2
[0125] The magnetic particle coating and the enzyme label were prepared in the same manner as in Example 1. The components of magnetic particle coating working solution (Ra), enzyme marker working solution (Rb) and test diluent (Rd) are the same as in Example 1.
[0126] Prepare the sample treatment solution (Rc) according to the composition in the table below
[0127]
[0128] Use the kit of this embodiment to detect HIV-1 / 2 antibody national reference substance and HIV p24 antigen national reference substance, negative and positive reference substance coincidence rate, precision, sensitivity and other index results are shown in Table 3 and Table 4. In addition, 10 HIV-1 / O antibody-positive samples were tested, and all 10 were positive.
[0129] Table 3 HIV-1 / 2 antibody national reference product test results
[0130]
[0131] Table 4 HIV p24 antigen national reference product test results
[0132]
[0133] In order to illustrate the influence of the pH value of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com