Phenyl anthracene silole derivative organic optoelectronic material and synthetic method thereof
An organic optoelectronic material and a synthesis method technology, applied in the field of chemistry, to achieve the effects of high catalytic activity and yield, good electroluminescence performance, and easy conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
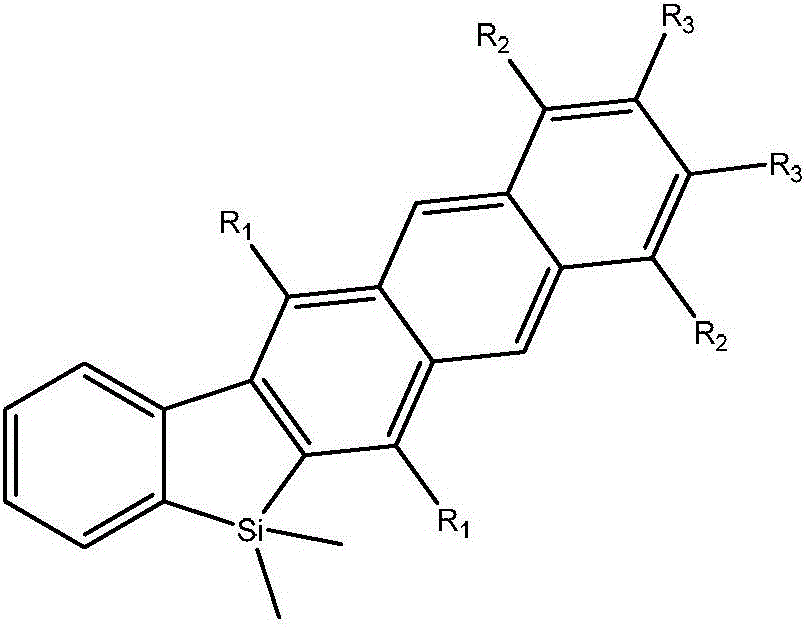
[0021] Example 1, the synthesis method of 1,2-diphenyl-5,8-dipropyl-6,7-dicarboxylic acid dimethyl ester-14,14-dimethyl-14 silfluorenone naphthalene, the 1, The structural formula of 2-diphenyl-5,8-dipropyl-6,7-dicarboxylic acid dimethyl ester-14,14-dimethyl-14 silfluorenonenaphthalene is shown in formula (1-10), which The synthetic method comprises the following steps:
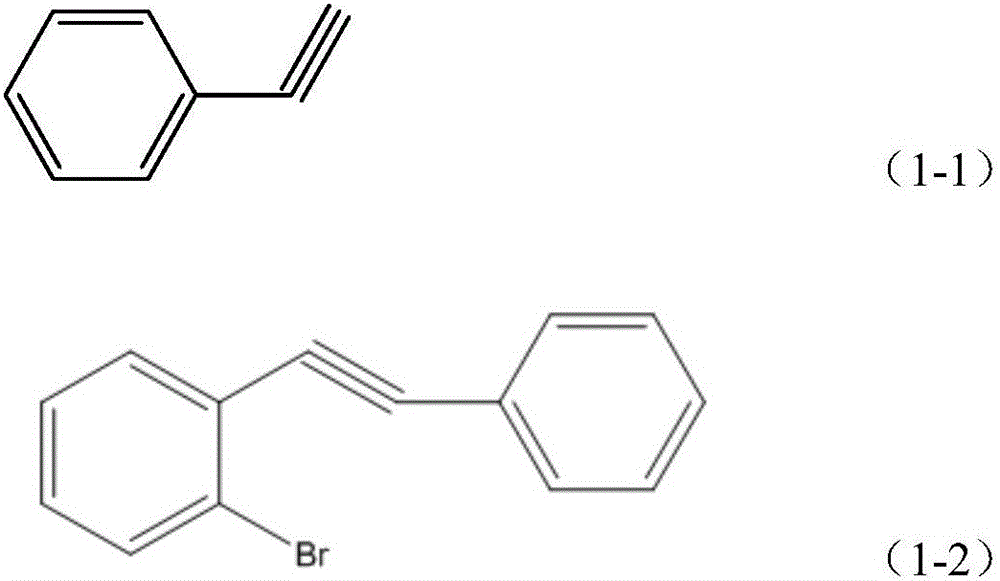
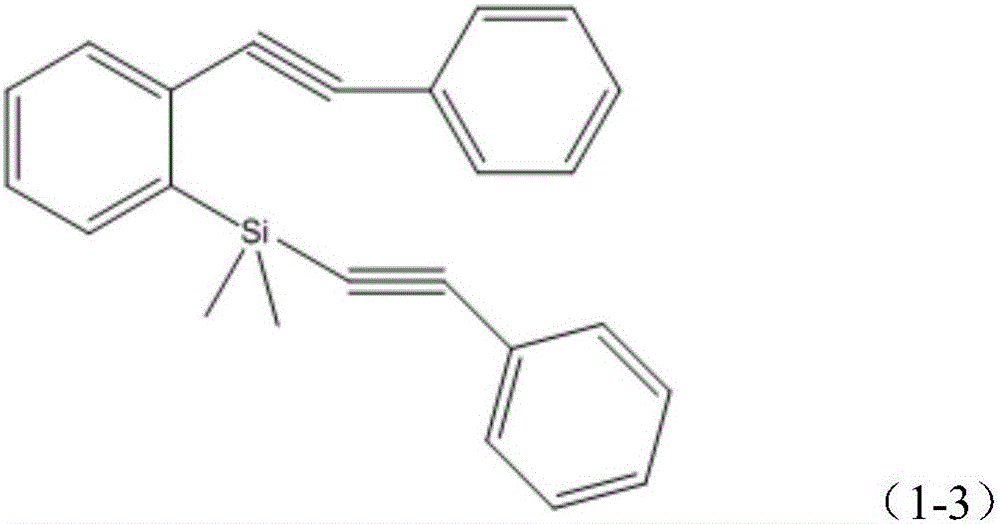
[0022] Step 1, preparation of phenylethynyl-(2-phenylethynyl)phenylsilane
[0023] Under nitrogen protection, 78mg (0.11mmol) bis(triphenylphosphine)-palladium dioxide, 1.06g (3.75mmol) o-bromoiodobenzene, 25mL triethylamine, 24mg (0.13mmol) CuI and 0.383g (3.75mmol) of phenylacetylene shown in structural formula (1-1), stirred at room temperature for 24h, filtered, the filtrate was extracted with ether, washed with water, dried, and rotary evaporated, and carried out column chromatography separation with pure n-hexane as the developing agent, 0.62 g (yield 64.3%) of 1-bromo-2-phenylethynylbenzene was obtai...
Embodiment 2
[0046] Example 2: Synthesis method of 1,5,8,12-tetraphenyl-6,7-dicarboxylic acid dimethyl-14,14-dimethylsilfluorenone naphthalene, the 1,5,8,12- The structural formula of tetraphenyl-6,7-dimethyl dimethyl-14,14-dimethylsilafluorene naphthalene is shown in formula (2-10), and its synthesis method comprises the following steps:
[0047] Step 1, preparation of phenylethynyl-(2-phenylethynyl)phenylsilane
[0048] Under nitrogen protection, add 316mg (0.45mmol) bis(triphenylphosphine)-palladium dioxide to 250mL reaction tube, 4.24g (15mmol) o-bromoiodobenzene, 100mL triethylamine, 95mg (0.5mmol) CuI, 1.532 g (15mmol) of phenylacetylene shown in structural formula (1-1), stirred at room temperature for 20h, filtered, the filtrate was extracted with ether, washed with water, dried, rotary evaporated, separated by column chromatography (developing agent is pure n-hexane), and 2.573 g (66.7% yield) 1-bromo-2-phenylethynylbenzene. The structural formula of the 1-bromo-2-phenylethynylb...
Embodiment 3
[0066] Example 3, the synthesis method of 1,2-diphenyl-5,8-dipropyl-6,7-dicarboxylic acid dimethyl ester-14,14-dimethyl-14 silfluorenone naphthalene, the 1, The structural formula of 2-diphenyl-5,8-dipropyl-6,7-dicarboxylic acid dimethyl ester-14,14-dimethyl-14 silfluorenonenaphthalene is shown in formula (1-10), which The synthetic method comprises the following steps:
[0067] Step 1, preparation of phenylethynyl-(2-phenylethynyl)phenylsilane
[0068] Under nitrogen protection, add 0.383mg (0.446mmol) bis(triphenylphosphine)-palladium dioxide, 4.24g (15mmol) o-bromoiodobenzene, 100mL triethylamine, 95mg (0.51mmol) CuI, into a 250mL reaction tube, 1.683g (16.48mmol) phenylacetylene, its structural formula is as shown in formula (1-1), stirred at room temperature for 22h, filtered, the filtrate was extracted with ether, washed with water, dried, rotary evaporated, separated by column chromatography (developing agent is pure n-hexane ), yielding 2.86 g (74.2%) of 1-bromo-2-ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com