Application of baohuoside I in preparation of drugs for treating asthma
A technology of baohuoside and its application is applied in the field of preparing drugs for treating allergic asthma, and can solve the problems that have not yet been seen, and the protein expression can be reduced.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
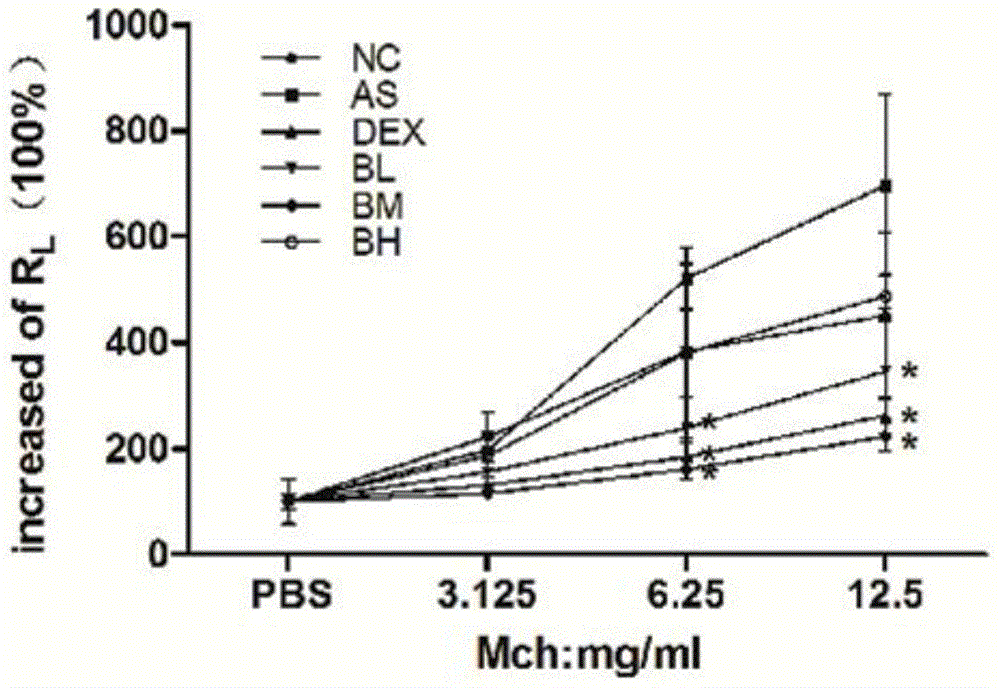
[0023] Example 1 Effect of Baohuoside I on AHR of Airway Hyperresponsiveness in Mouse Asthma Model
[0024] The baohuoside I monomer of the formula (I) is obtained by separating and preparing the baohuoside I monomer of the formula (I) through the commercial channel or using the crude extract of Epimedium herba as raw material, and its molecular formula is: C 27 h 30 o 10 , Molecular weight: 514.5211.
[0025] 60 female BALB / c mice aged 7 weeks in healthy and clean grade, with a body weight of 16-18 g (provided by Shanghai Xipuerbikai Experimental Animal Co., Ltd.), were randomly divided into 6 groups, 10 in each group, namely the normal control group ( NC), asthma model group (AS), dexamethasone intervention group (DEX), baohuoside Ⅰ low, medium, and high dose intervention groups (BL, BM, BH); mice were given a one-time intraperitoneal injection from day 0 0.2ml of physiological saline suspension of 20ug ovalbumin and 2mg aluminum hydroxide was used to make the mice in a s...
Embodiment 2
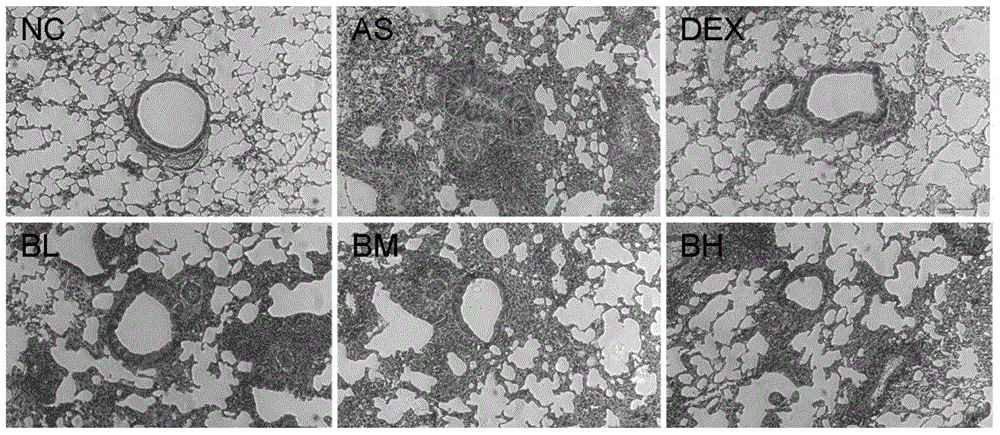
[0029] Example 2 Effect of baohuoside I on airway inflammation in ovalbumin-induced mouse asthma model
[0030] The right lung tissue of the experimental mice was taken, after conventional treatment, soaked and fixed in 4% neutral formaldehyde, embedded in paraffin, stained with HE, and observed with a light microscope for inflammatory changes; the results showed that the airways and surrounding lung tissues of the mice in the asthma model group were visible The infiltration of inflammatory cells such as neutrophils, lymphocytes, macrophages and eosinophils; the intervention of baohuoside I can significantly reduce the inflammatory changes such as inflammatory cell infiltration and alveolar congestion in the airway wall of mice.
Embodiment 3
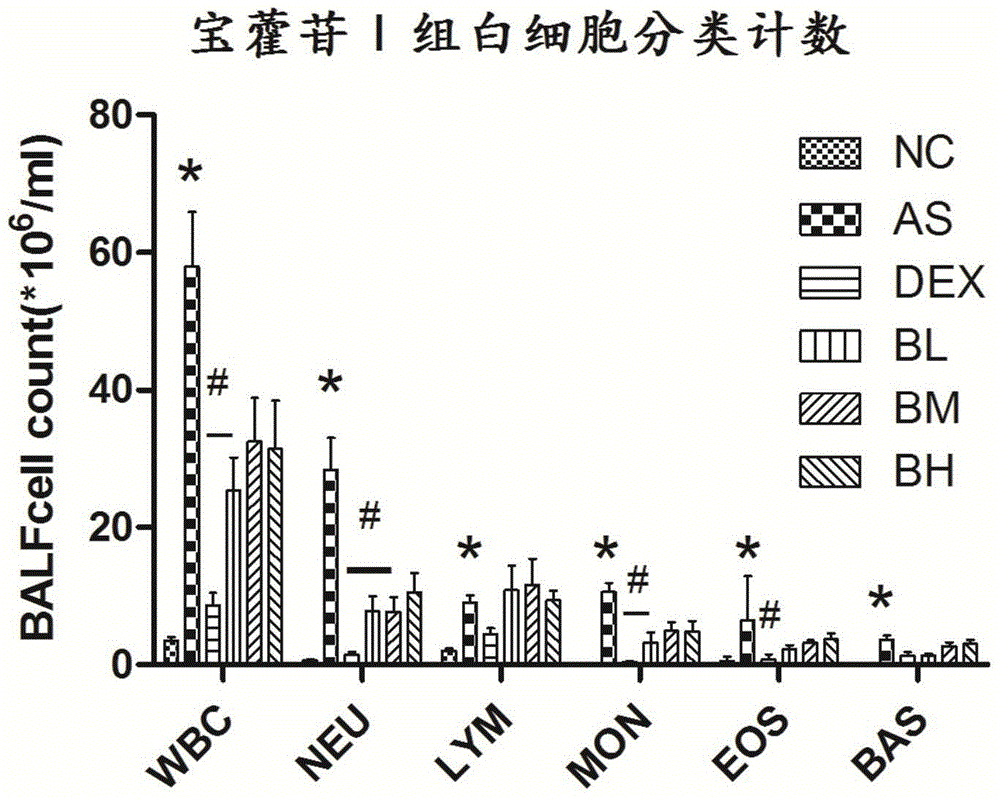
[0031] Example 3 Effect of Baohuoside I on Inflammatory Cells in Ovalbumin-Induced Mouse Asthma Model BALF
[0032] After the experimental mice completed the lung function measurement, insert a tracheal lavage needle into the left bronchus, open the chest and ligate the right main bronchus and right lung, and use 0.3ml PBS to lavage the left lung twice; the recovered BALF was 800g at 4°C After ×10min centrifugation, the cell pellet was resuspended with 100ul PBS solution, and the white blood cell differential count was carried out; the results showed that, compared with the normal group, the total white blood cell count (WBC), neutrophil (NEU), lymphocyte ( LYM), monocytes (MON), eosinophils (EOS), and basophils (BAS) were significantly increased; Baohuoside Ⅰ low-dose group could significantly reduce WBC, NEU, MON and EOS cells in BALF Quantity (p<0.05).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com