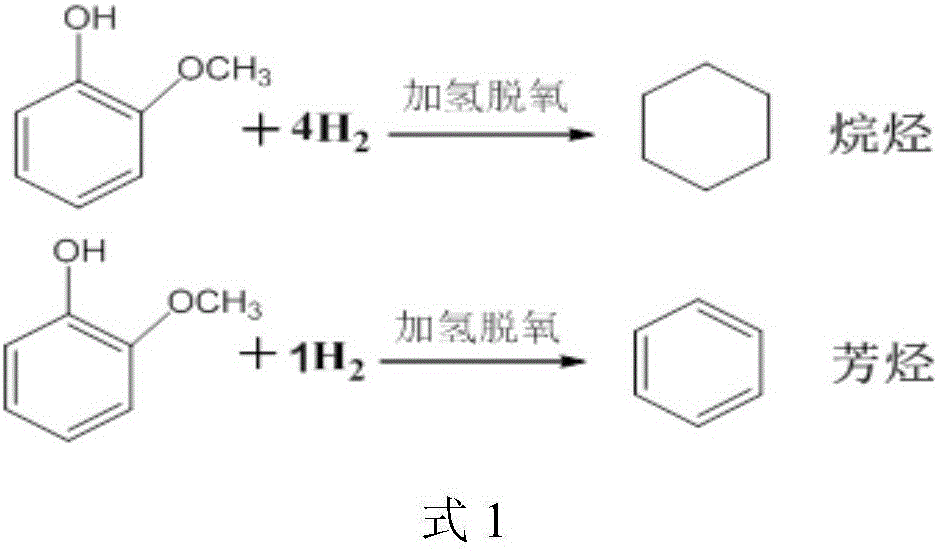
Method for preparation of aromatic hydrocarbon by catalyzing hydrodeoxygenation of monocyclic phenolic platform compound under low hydrogen partial pressure
A hydrodeoxygenation and compound technology, which is applied in catalyst activation/preparation, hydrocarbon production from oxygen-containing organic compounds, carbon compound catalysts, etc., can solve the problems of expensive catalysts, high hydrogen pressure, easy carbon deposition and deactivation, etc., to achieve Effect of low hydrogen partial pressure, improved selectivity, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: MoO 3 Catalyst preparation
[0023] 0.01 mol of (NH 4 ) 6 Mo 7 o 24 .4H 2 O was dissolved in deionized water, and 10 times the molar equivalent of hydrochloric acid solution (0.1 molar hydrochloric acid solution) was added dropwise under magnetic stirring to form a white flocculent precipitate; the precipitate was placed in an oven at 105°C after standing for aging, filtering, and washing Drying in medium for 12h, then calcining at 500°C in air atmosphere in muffle furnace for 5 hours and then in reducing atmosphere (5vol.%H 2 +95vol%N 2 ) at 450-800°C for 2-24 hours.
Embodiment 2
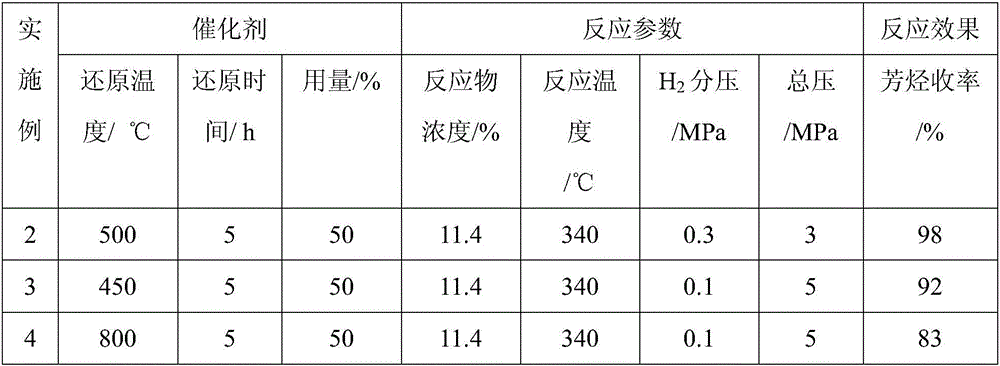
[0025] The 1.0g MoO obtained in Example 1 was reduced at 500°C for 5h 3 Catalyst, the phenol of 2.0g (the mass concentration of phenol is 11.4%) and the n-octane of 25.0ml are put into the autoclave of 100ml band stirrer, with N 2 Replace the air in the kettle, fill with H 2 After boosting to 0.3MPa, fill with N 2 Until the pressure in the reactor reached 3.0 MPa, the reactor was sealed. Turn on the stirring paddle (700 rpm), raise the temperature of the reactor to 340° C. at a heating rate of 10° C. / min, and start timing the reaction. The reaction time is 5h. The yield of product benzene was 98% (see Table 1).
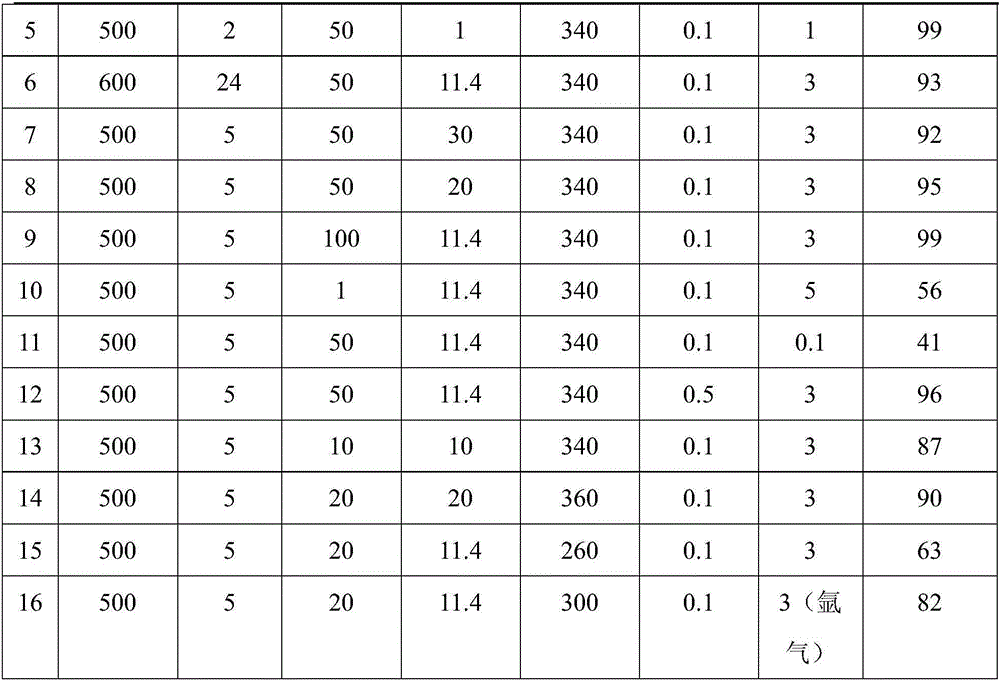
Embodiment 3- Embodiment 16
[0027] With reference to Example 2, the difference is that the reaction time is 6h, and the reduction conditions (reduction temperature and time), consumption, and reaction parameters of the catalyst are different, and the gas phase is H in Example 16. 2 The mixture of +Ar, see Table 1, and the product yield also sees Table 1.
[0028] Table 1
[0029]
[0030]
[0031] In Table 1, reactant: phenol; solvent: n-octane; reaction time: 6h; stirring speed: 700rpm. In Example 2-Example 15, the gas phase is H 2 +N 2 The mixture; Gas phase is H in embodiment 16 2 +Ar mixture.
[0032]It can be seen from the examples that the method for preparing aromatics by catalytic monocyclic phenolic platform compound hydrodeoxygenation is carried out in a mixed gas of hydrogen and nitrogen or argon, preferably under high nitrogen partial pressure and low hydrogen partial pressure, and hydrogen partial pressure The pressure is 0.1-0.3Mpa. ; The reaction temperature is 300-360°C. Witho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com