PEGylated Fe3+/ PEI genetic vector based on functional peptide R9 modification and preparation method and application of PEGylated Fe3+/ PEI genetic vector
A gene carrier and functional peptide technology, applied in the medical field, can solve the problems of poor targeting of polyethyleneimine, strong cytotoxicity, and easy dissociation, so as to improve nuclear delivery capacity, reduce cytotoxicity, and improve transfection efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 PEG-Fe modified by polypeptide R9 3+ Preparation and functional verification of / PEI gene carrier (1)
[0045] 1. PEG-Fe 3+ Synthesis of / PEI
[0046] 20 μL FeCl 3 ·6H 2 O (250mg / mL), 30μL bPEI (250mg / mL), 10μL vinyl sulfone (10mg / mL), 15μL PEG-NHS (Mw=5000, 10mg / mL) were sequentially added to 1mL Tween 80 cyclohexane solution (250mg / mL mL), room temperature, fully stirred for 12h. After the reaction, add excess ethanol to the emulsion, centrifuge at 10,000r / min for 1 hour, remove the supernatant carefully, and disperse the precipitate in ethanol again by sonication. Pure water dissolves the precipitate, and the result is PEG-Fe 3+ / PEI polymer solution.
[0047] 2. Synthesis of functional peptide R9
[0048] The sequence of the functional peptide R9 is Arg-Gly-Asp-Cys-Lys-Lys-Lys-Arg-Lys, which was synthesized by Shanghai Gil Biochemical Co., Ltd. by solid-phase method.
[0049] 3. PEG-Fe modified by polypeptide R9 3+ Preparation of / PEI
[0050] S...
Embodiment 2
[0055] Example 2 PEG-Fe modified by polypeptide R9 3+ Preparation and functional verification of / PEI gene carrier (2)
[0056] PEG-Fe 3+ The preparation, cytotoxicity test, and in vitro transfection experiment of / PEI-R9 are the same as in Example 1, except that the PEG-Fe prepared in this example 3+ / PEI-R9, where PEI and Fe 3+ The molar ratio of PEI is 1:6, the molecular weight of PEI is 5KDa, and the molar ratio of PEGylated FE3+ / PEI to polypeptide R9 is 1:5.
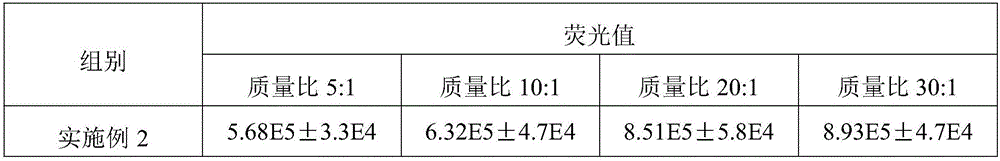
[0057] Cytotoxicity test results showed that: PEG-Fe 3+ / PEI-R9 is almost non-toxic, the PEG-Fe of the present embodiment 3+ / PEI-R9 has low cytotoxicity, ensuring that PEG-Fe 3+ / The feasibility of PEI-R9 as a gene carrier. The results of in vitro transfection experiments are shown in Table 1.
Embodiment 3
[0058] Example 3 PEG-Fe modified by polypeptide R9 3+ Preparation and functional verification of / PEI gene carrier (3)
[0059] PEG-Fe 3+ The preparation, cytotoxicity test, and in vitro transfection experiment of / PEI-R9 are the same as in Example 1, except that the PEG-Fe prepared in this example 3+ / PEI-R9, where PEI and Fe 3+ The molar ratio of the PEGylated FE3+ / PEI to the polypeptide R9 is 1:10, the molecular weight of PEI is 25KDa, and the molar ratio of the PEGylated FE3+ / PEI to the polypeptide R9 is 1:10.
[0060] Cytotoxicity test results showed that: PEG-Fe 3+ / PEI-R9 is almost non-toxic, the PEG-Fe of the present embodiment 3+ / PEI-R9 has low cytotoxicity, ensuring that PEG-Fe 3+ / The feasibility of PEI-R9 as a gene carrier. The results of in vitro transfection experiments are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com