Method for producing 2-acetylamino-6-nitrobenzoic acid by sodium chloride reduction
A technology of nitrobenzoic acid and acetamido, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problem that the quality cannot meet the requirements of drug and dye production, the control of the end point of acylation is difficult, and the process is complicated and other problems, to achieve the effect of not easy to oxidize, simple equipment and operation, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The present invention will be further described below:
[0020] The present invention comprises the following steps:
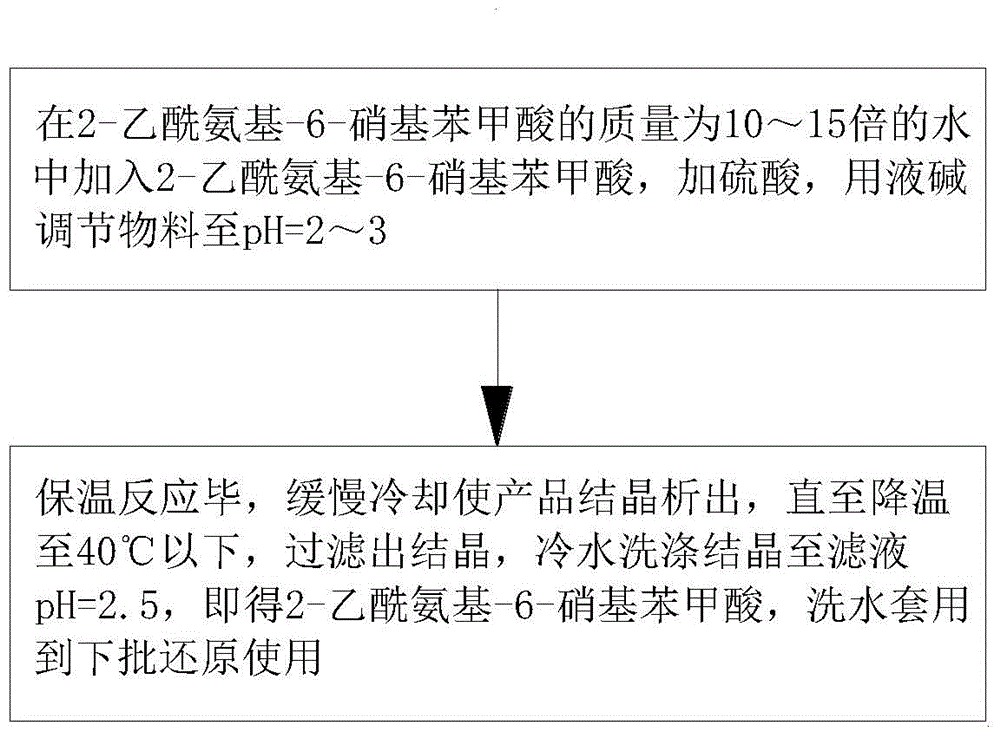
[0021] like figure 1 As shown, in step a, 2-acetylamino-6-nitrobenzoic acid is added to water whose mass is 10 to 15 times that of 2-acetylamino-6-nitrobenzoic acid, sulfuric acid is added, and the material is adjusted to pH with liquid caustic soda =2~3; be warming up to 70~89 DEG C, add 45~75% sodium chloride solution dropwise at a uniform speed with the time of 0.5-1 hour, dropwise, insulated reaction 3 hours under this temperature; Described amino is 1 The amino group of ~3 ammonia atoms, the ratio of the amount of described reaction substance is 2-acetamido-6-nitrobenzoic acid: sodium chloride=3:6.5~8.0, the sulfuric acid that adds when preparing buffer solution is converted into The mass ratio of 100% sodium chloride is sulfuric acid: sodium chloride=0.1~3:2, described 45~75% sodium chloride solution, both available solid sodium chloride prepara...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com