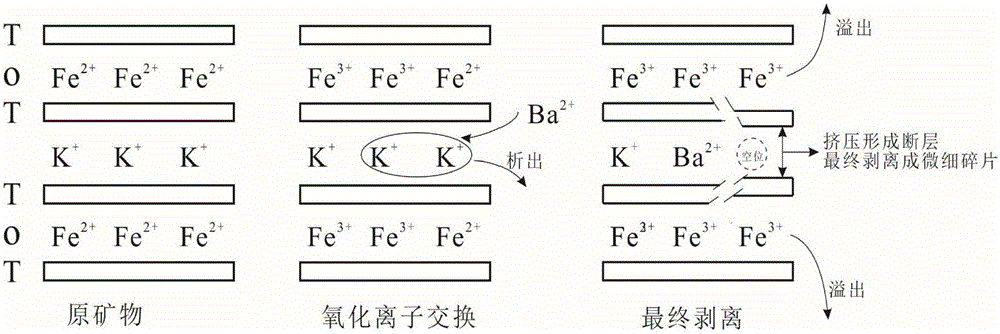
Method for decomposing biotite by using dilute hydrochloric acid and barium nitrate
A technology of dilute hydrochloric acid and barium nitrate, applied in aluminum silicate, silicate and other directions, can solve the problems of high harm and high equipment requirements, and achieve the effects of low equipment requirements, simple process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Use a ball mill to pulverize biotite ore and pass through a 200-mesh sieve to obtain biotite powder.
[0024] (2) Mix 0.5g of biotite powder with 100mL of dilute hydrochloric acid with pH=2, add 20g of barium nitrate thereto and mix well. The mixed solution was placed in a constant temperature water bath at 60° C. to stir for reaction (the mixed solution at this temperature was a saturated solution of barium nitrate), and after 5 hours, it was filtered and dried to obtain a solid. Under the same conditions, accurately weigh the solid obtained above and add it to dilute hydrochloric acid, keeping the ratio of the two at 1g:200ml. According to the content of dilute hydrochloric acid, the added barium nitrate makes the mixed solution saturated with barium nitrate at the reaction temperature, reacts in a constant temperature water bath at 60°C and filters to obtain a solid. Repeat the above operation 3 times.
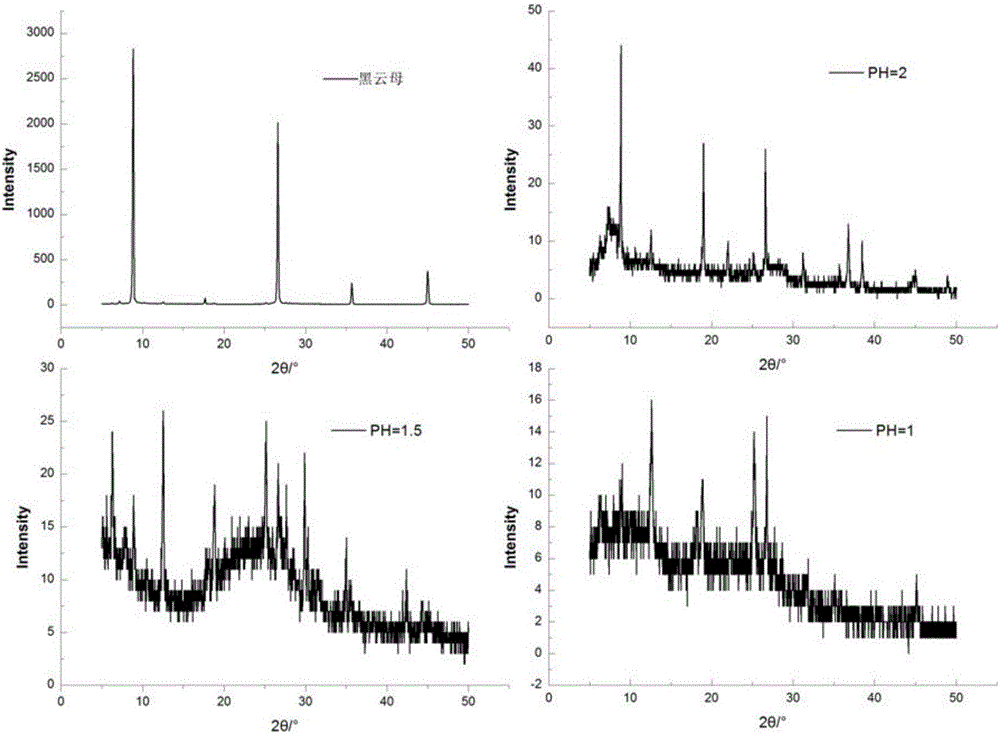
[0025] Analyze the last solid insoluble matter with X-ray...
Embodiment 2
[0027] (1) Use a ball mill to pulverize biotite ore and pass through a 200-mesh sieve to obtain biotite powder.
[0028] (2) Mix 0.5g of biotite powder with 100mL of dilute hydrochloric acid with pH=1.5, add 20g of barium nitrate therein and mix well. The mixed solution was placed in a constant temperature water bath at 60° C. to stir for reaction (the mixed solution at this temperature was a saturated solution of barium nitrate), and after 5 hours, it was filtered and dried to obtain a solid. Under the same conditions, accurately weigh the solid obtained above and add it to dilute hydrochloric acid, keeping the ratio of the two at 1g:200ml. According to the content of dilute hydrochloric acid, the added barium nitrate makes the mixed solution saturated with barium nitrate at the reaction temperature, reacts in a constant temperature water bath at 60°C and filters to obtain a solid. Repeat the above operation 3 times.
[0029] Analyze the last solid insoluble matter with X-r...
Embodiment 3
[0031] (1) Use a ball mill to pulverize biotite ore and pass through a 200-mesh sieve to obtain biotite powder.
[0032] (2) Mix 0.5g of biotite powder with 100mL of dilute hydrochloric acid with pH=1, add 20g of barium nitrate thereto and mix well. The mixed solution was placed in a constant temperature water bath at 60° C. to stir for reaction (the mixed solution at this temperature was a saturated solution of barium nitrate), and after 5 hours, it was filtered and dried to obtain a solid. Under the same conditions, accurately weigh the solid obtained above and add it to dilute hydrochloric acid, keeping the ratio of the two at 1g:200ml. According to the content of dilute hydrochloric acid, the added barium nitrate makes the mixed solution saturated with barium nitrate at the reaction temperature, reacts in a constant temperature water bath at 60°C and filters to obtain a solid. Repeat the above operation 3 times.
[0033] Analyze the last solid insoluble matter with X-ray...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com