Solid preparation for coating agent, and film and coated solid preparation formed from same
A technology of solid preparation and coating agent, which can be used in sugar-coated pills, pill delivery, inorganic non-active ingredients, etc., and can solve the problems of unstable oxygen and water vapor.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0071] Hereinafter, in order to demonstrate the excellent effects of the present invention, examples are used to illustrate, but the present invention is not limited thereby.
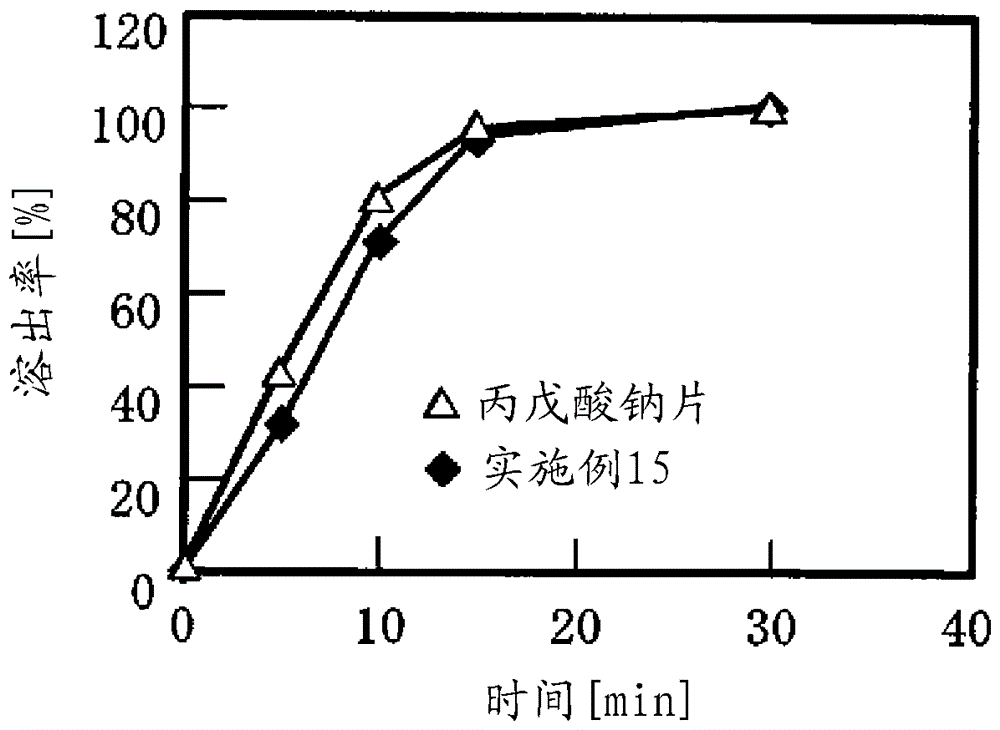
[0072] (Measurement of oral disintegration time)
[0073] Oral disintegration time was measured by 3 testers consisting of healthy adult males and females. The oral disintegration time of the core tablet was subtracted from the oral disintegration time of the orally disintegrable coated tablet, and the obtained time was calculated as the oral disintegration time of the coating.
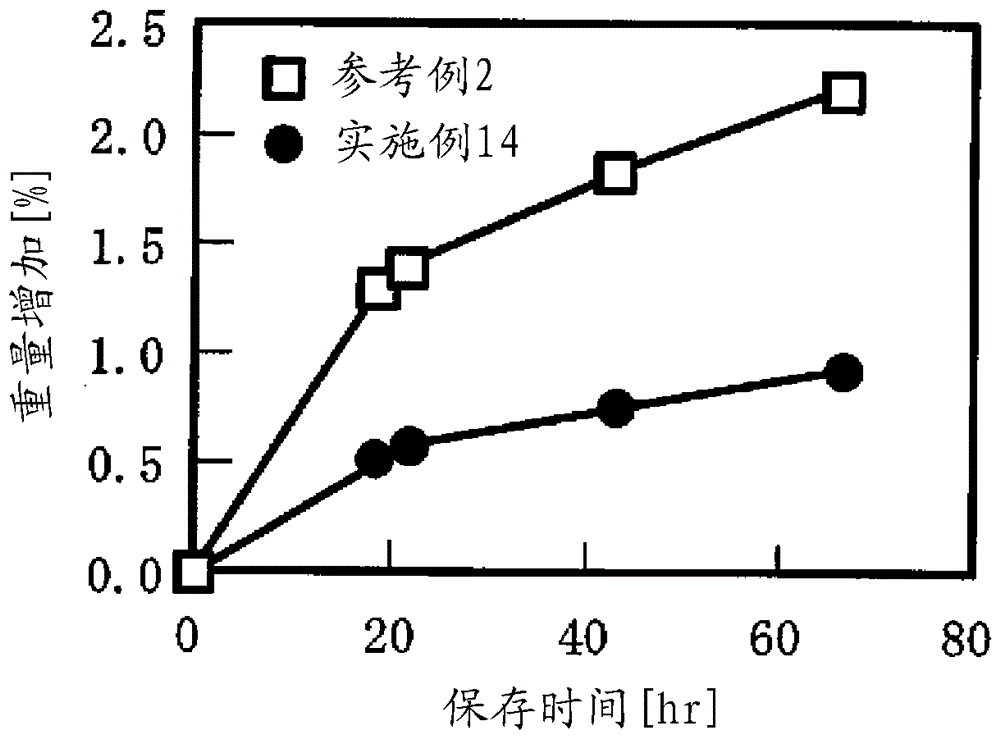
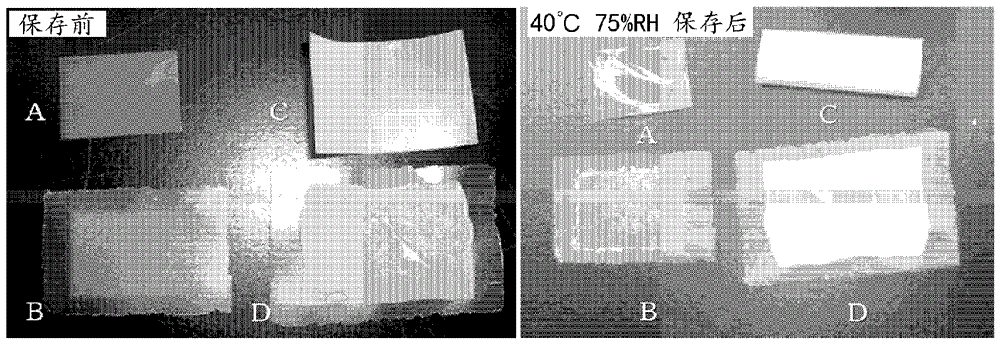
[0074] (Cracks in the film under humidified conditions)
[0075] After storing at 40° C. and 75% RH for 1 day, the tablet was visually observed to evaluate whether or not the film was cracked.
[0076] (Measurement of Water Vapor Permeability Using Single Membrane)
[0077] The coating agent is repeatedly sprayed on the release film and dried to form a single film. The water vapor permeability is measured by partially modif...
reference example 1
[0089] (Reference Example 1) Production of Orally Disintegrating Tablets (Core Tablets)
[0090] 122.6225 parts by weight of mannitol (Pearitol (registered trademark); Roketto Japan Co., Ltd.) (hereinafter abbreviated as "parts", the same unless otherwise specified), 0.0025 parts of nafurphine hydrochloride, thiosulfuric acid 0.225 parts of sodium hydrate and 6.5 parts of crospovidone (Kollidon (registered trademark) CL; BASF Corporation) were put into a fluidized bed granulator (FLO-5; FLOINT INDUSTRIAL CO., LTD.) to produce granulated granules. Next, it was treated with Comil (197S; Powrek Co., Ltd.) to obtain sized pellets. 0.65 parts of magnesium stearate (Ohira Chemical Industry Co., Ltd.) was mixed with 129.35 parts of sized granules to obtain granules for tableting. The granules for tabletting were prepared into 130 mg 7 mmφSR tablets using a tablet press (Correct 19; Kikusui Seisakusho). The oral disintegration time of this tablet is 9 seconds.
reference example 2
[0091] (Reference Example 2) Production of hygroscopic orally disintegrating tablet (core tablet)
[0092] 26.3 parts of mannitol (Pearitol (registered trademark); Rocket Japan Co., Ltd.), 1.5 parts of hydroxypropyl cellulose (HPC-L; Nippon Soda Co., Ltd.), N-[(5R,6R,14S)-17-(cyclo 0.2 part of propylmethyl)-4,5-epoxy-3,14-dihydroxymorphinan-6-yl]phthalimide was charged into the fluidized bed granulator to produce granulated granules. Next, Comil is used for treatment to obtain sized granules. With 28 parts of sized granules, 94.2 parts of powdered sorbitol (NEOSORB (registered trademark); Rocket Japan Co., Ltd.) and 6.5 parts of crospovidone were mixed, and 1.3 parts of magnesium stearate were mixed to obtain granules for tableting. The granules for tabletting were formed into 130 mg of 7 mmφWR tablets using a tablet machine. The oral disintegration time of this tablet was 41 seconds.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com