Immobilization method of xylanase and immobilized xylanase
A xylanase and modification technology, applied in the field of enzyme engineering, can solve the problems of high price of enzymes, limited application, difficult recovery, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Weigh APTES modified Fe 3 o 4 @SiO 2 Nanoparticles 1.0057g was placed in 100mL (pH=5.5) of acetic acid-sodium acetate buffer solution, added 4% (v / v) 19.05mL concentration of 25wt% glutaraldehyde solution, mechanically stirred in a water bath at 25°C, 180rpm After stirring for 4 hours, APTES modified Fe 3 o 4 / SiO 2 Nanoparticles loaded with glutaraldehyde;
[0054] Add 0.1295 g of xylanase dissolved in acetic acid-sodium acetate buffer solution, and mechanically stir at 180 rpm for 3 hours to obtain immobilized xylanase;
[0055] Magnetically separate, wash with acetic acid-sodium acetate buffer solution 3 to 4 times, and finally use acetic acid-sodium acetate buffer solution to make up to 100mL; put it in the refrigerator for later use.
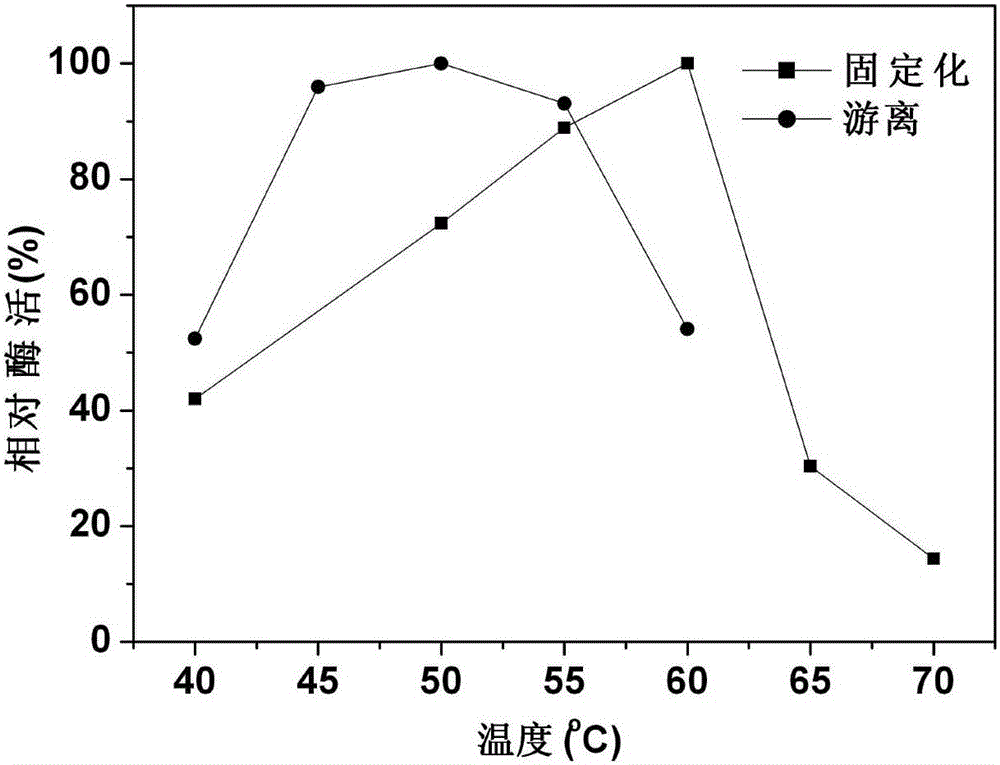
[0056]Test the impact of different influencing factors on the enzymatic activity of the immobilized xylanase obtained in the present embodiment and the free state xylanase:
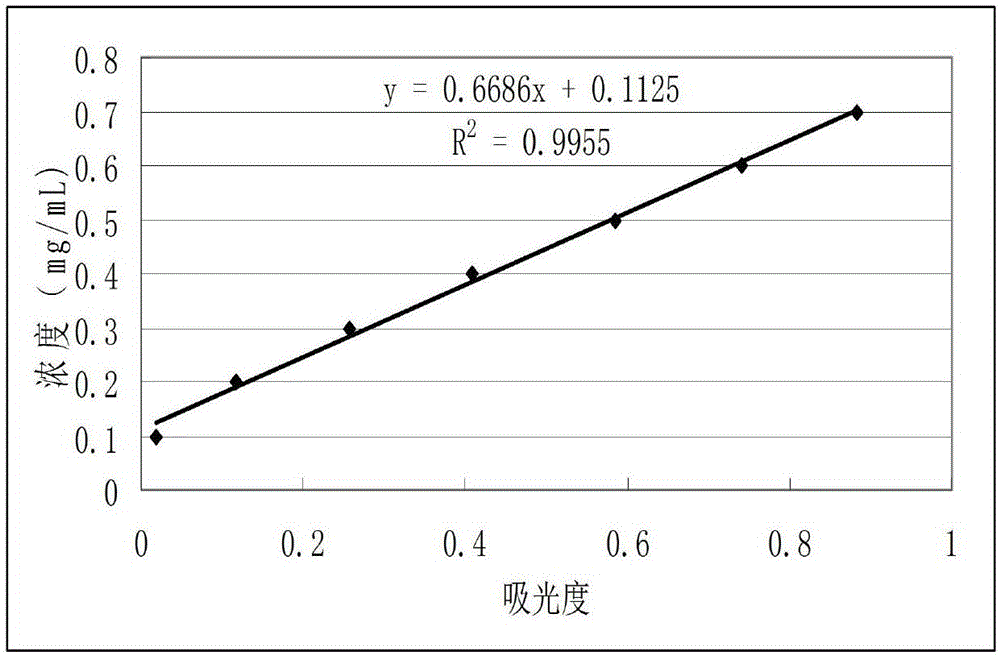
[0057] 1. Preparation of solutions and samples
[...
Embodiment 2
[0107] Weigh APTES modified Fe 3 o 4 @SiO 2 Nanoparticle 5g is placed in the acetic acid-sodium acetate buffer solution of 150mL (pH=5.5), add 4% (v / v) 95mL concentration and be 25wt% glutaraldehyde solution, carry out mechanical stirring in 25 ℃ of water baths, 180rpm stirs 4 hours, get APTES modified Fe 3 o 4 / SiO 2 Nanoparticles loaded with glutaraldehyde;
[0108] Add 0.65 g of xylanase dissolved in acetic acid-sodium acetate buffer solution, and mechanically stir at 180 rpm for 3 hours to obtain immobilized xylanase;
[0109] Magnetically separate, wash with acetic acid-sodium acetate buffer solution 3 to 4 times, and finally use acetic acid-sodium acetate buffer solution to make up to 100mL; put it in the refrigerator for later use.
[0110] The immobilized xylanase obtained in the present embodiment is tested for performance using the experimental means in Example 1, and the results are as follows:
[0111] 60°C is the best pretreatment temperature for immobilize...
Embodiment 3
[0118] Weigh APTES modified Fe 3 o 4 @SiO 2 Nanoparticle 15g is placed in the acetic acid-sodium acetate buffer solution of 500mL (pH=5.5), add 4% (v / v) 285mL concentration and be 25wt% glutaraldehyde solution, carry out mechanical stirring in 25 ℃ of water baths, 180rpm stirs 4 hours, get APTES modified Fe 3 o 4 / SiO 2 Nanoparticles loaded with glutaraldehyde;
[0119] Add 1.95 g of xylanase dissolved in acetic acid-sodium acetate buffer solution, and mechanically stir at 180 rpm for 3 hours to obtain immobilized xylanase;
[0120] Magnetically separate, wash with acetic acid-sodium acetate buffer solution 3 to 4 times, and finally use acetic acid-sodium acetate buffer solution to make up to 300mL; put it in the refrigerator for later use.
[0121] The immobilized xylanase obtained in the present embodiment is tested for performance using the experimental means in Example 1, and the results are as follows:
[0122] 60°C is the best pretreatment temperature for immobili...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com