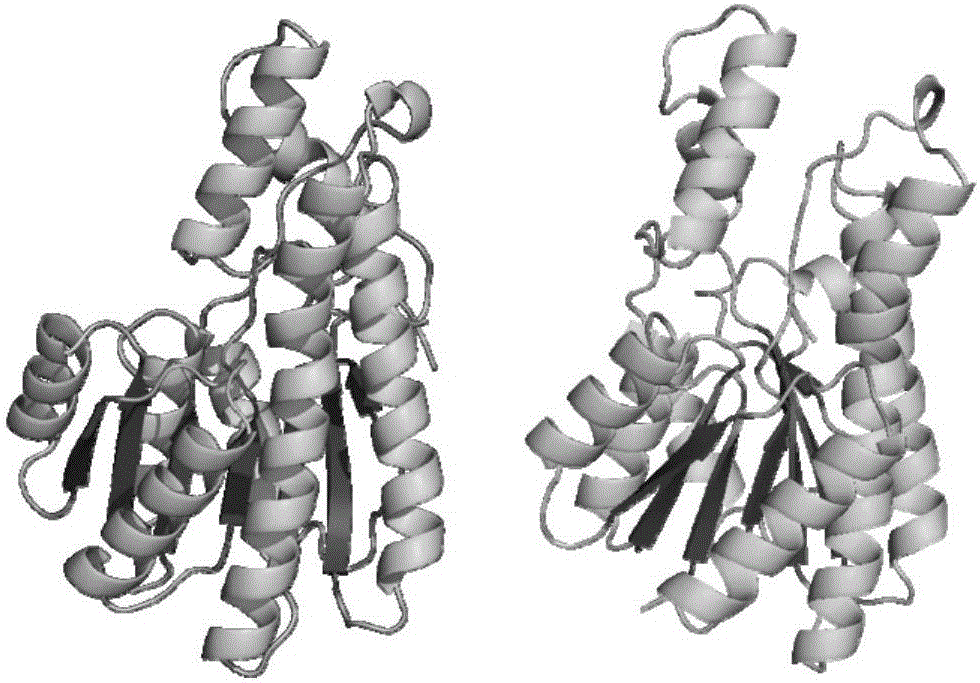Immobilized sorbitol dehydrogenase as well as immobilization method and application of immobilized sorbitol dehydrogenase
A sorbitol dehydrogenase and volume technology, which is applied in the directions of immobilized enzymes, biochemical equipment and methods, oxidoreductases, etc., can solve the problems of groping blanks in production process conditions, and achieves the improvement of the number of reuses, the method is simple, the mechanical high intensity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 Preparation of free sorbitol dehydrogenase
[0033] Referring to the method disclosed in the invention patent CN105154457A, prepare Escherichia coli engineering bacteria containing sorbitol dehydrogenase, induce expression for 15 h, centrifuge the bacterial liquid at 4 °C and 8 000 r / min for 15 min, discard the supernatant, and collect the The cells were washed twice with sodium phosphate buffer (pH 7.0) and suspended in the same buffer, and the cells were broken using a high-pressure homogenizer (-20°C, 8.0×107Pa). After centrifugation at 4 °C and 10 000 r / min for 30 min, the cell disruption solution was discarded, and the supernatant was the crude enzyme solution of sorbitol dehydrogenase.
Embodiment 2
[0034] Example 2 Computer Simulation Assisted Immobilized Enzyme Condition Optimization
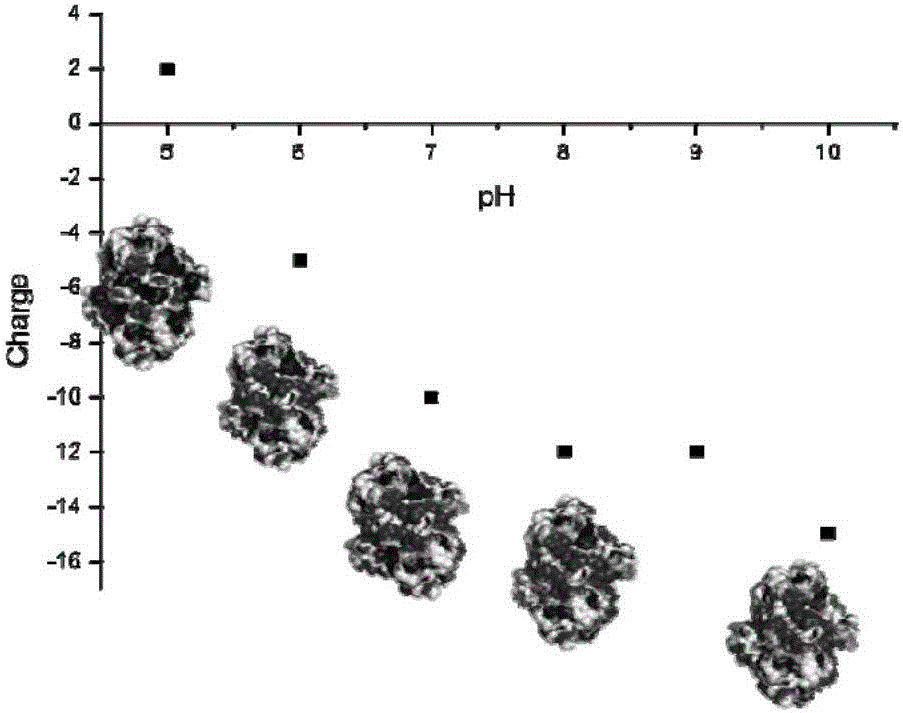
[0035] Using the online tool swiss-model (https: / / swissmodel.expasy.org / ), and using PDB:1K2W as a template, carry out 3D structural homology modeling on the amino acid sequence of sorbitol dehydrogenase obtained in Example 1, and Its volume size is predicted to be 7 × 7 × 2.5 nm (Fig. 1). Submit the 3D homology model of sorbitol dehydrogenase to the online server PDB2PQR (http: / / nbcr-222.ucsd.edu / pdb2pqr_2.1.1 / ), analyze its surface potential under different pH environmental conditions, and predict the appropriate The pH range of the immobilization is 4~5.5 (Figure 2).
Embodiment 3
[0036] Example 3 Immobilization operation of sorbitol dehydrogenase
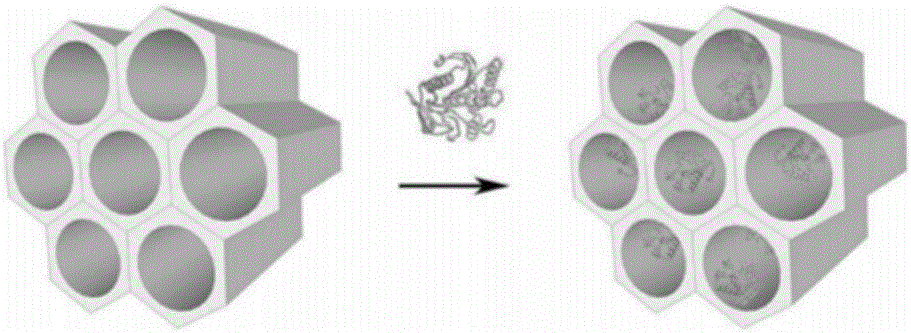
[0037] Prepare 2 mg / mL SBA15 mesoporous material (Nanjing Xianfeng Nano Material Technology Co., Ltd.) with sodium acetate buffer at pH 5.5. After ultrasonication for 15 min, stir magnetically for 30 min to obtain evenly dispersed mesoporous materials. To the material solution, add the free sorbitol dehydrogenase in Example 1, the final concentration is 1 mg / mL, stir or shake at a low speed for 0.25 h at room temperature, take out and filter, rinse with sodium acetate buffer, and place at 4°C Preserved, the protein immobilization capacity is 545 mg / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com