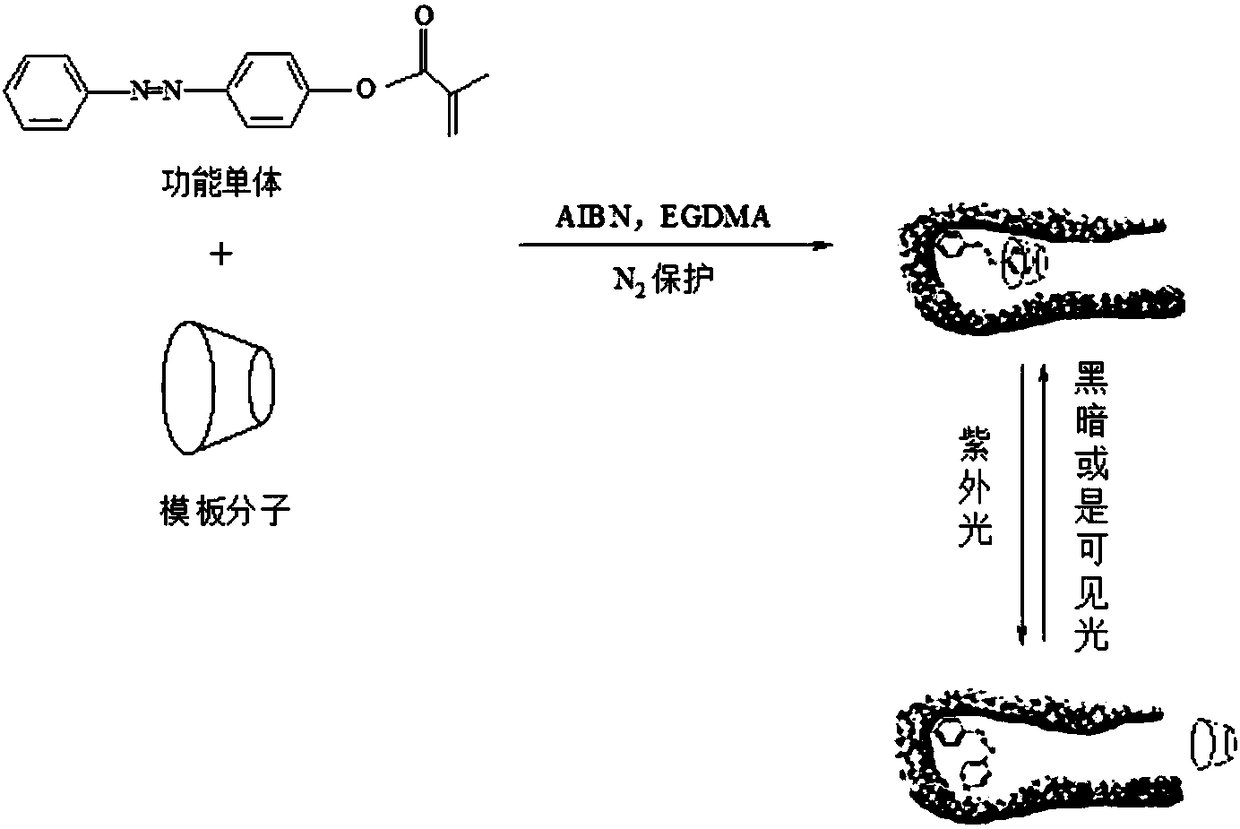
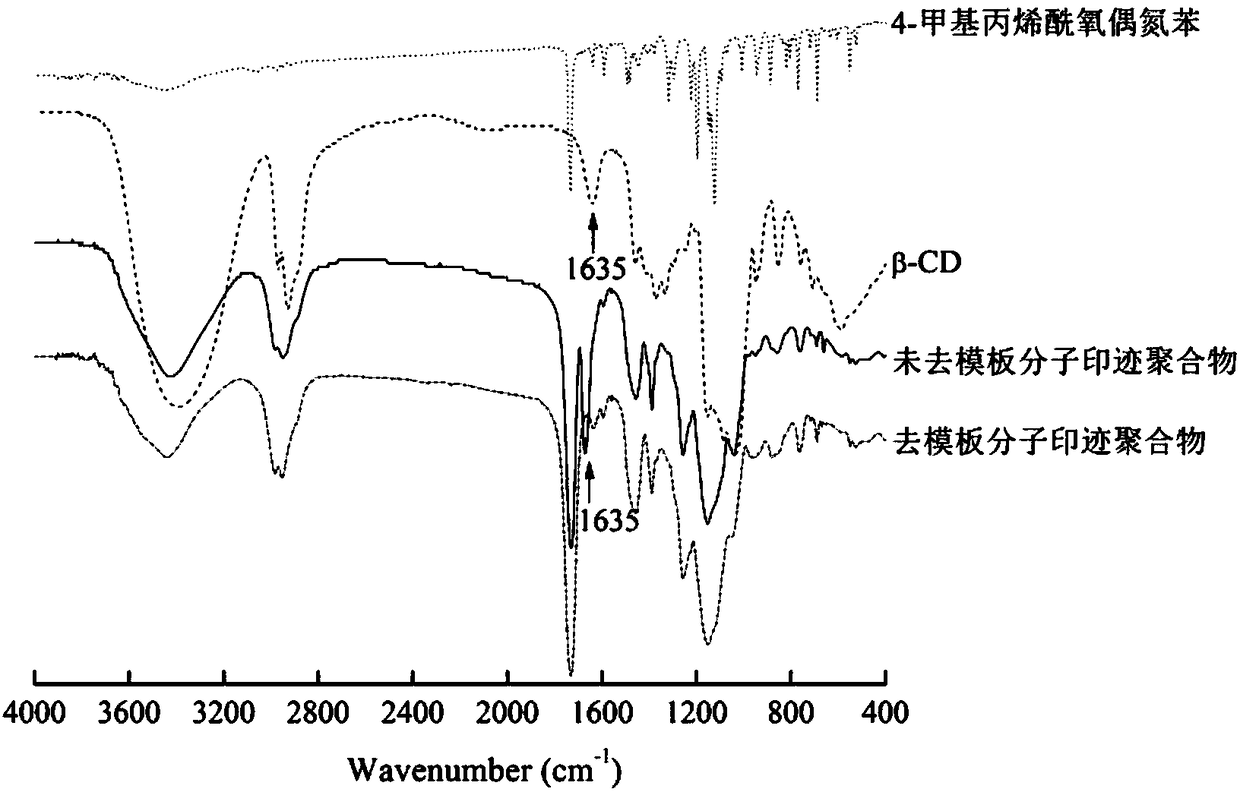
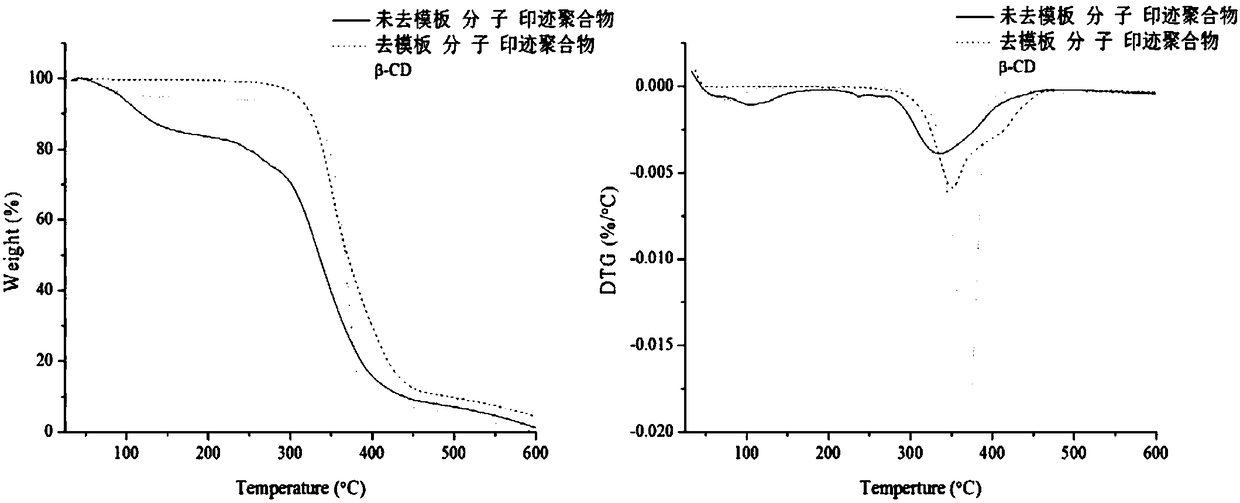
Preparation method of photoresponsive molecularly imprinted material for selective separation and purification of cyclodextrin and its derivatives
A separation and purification, molecular imprinting technology, applied in the field of chemistry and material and molecular separation, can solve the problems of restricting the industrial production of cyclodextrin, low industrial yield, high price, etc., to achieve excellent light response characteristics, remarkable separation effect, use long life effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Aniline (15g, 162mmol), 10mL of water and 10mL of concentrated hydrochloric acid with a concentration of 12mol / L were mixed and stirred for 10min, then NaNO dissolved in 20mL of water was added dropwise 2 (12g). After continuing to stir for 20 min under ice-bath conditions, a phenol solution dissolved in 40 mL of 8% NaOH was added dropwise. After the dropwise addition, keep the ice bath and continue to stir for 40 min. After the reaction, filter with suction, wash the filter cake with water, and then recrystallize with a mixture of ethanol and water with a volume ratio of 1:1 to obtain a deep orange-red product 4-hydroxyazobenzene;
[0040] (2) Dissolve 0.15g N,N-dimethylaminopyridine, 2.0g triethylamine and 2.0g 4-hydroxyazobenzene in 100.0mL acetonitrile, place the mixture in an ice-water bath, and slowly add 2.0 g methacryloyl chloride and 5.0mL acetonitrile mixed solution, moved to an oil bath at 35°C to react for 48h, cooled to room temperature, added 30.0mL ...
Embodiment 2
[0043] (1) Aniline (15g, 162mmol), 80mL of water and 30mL of concentrated hydrochloric acid with a concentration of 11mol / L were mixed and stirred for 20min, then NaNO dissolved in 30mL of water was added dropwise 2 (13g). After continuing to stir for 40 min under ice-bath conditions, a phenol solution dissolved in 90 mL of 10% NaOH was added dropwise. After the dropwise addition, the ice bath was kept and stirring was continued for 300 min. After the reaction, filter with suction, wash the filter cake with water, and then recrystallize with a mixture of ethanol and water with a volume ratio of 1:1 to obtain a deep orange-red product 4-hydroxyazobenzene;
[0044] (2) Dissolve 0.75g N,N-dimethylaminopyridine, 20.0g triethylamine and 6.0g 4-hydroxyazobenzene in 150.0mL acetonitrile, place the mixture in an ice-water bath, and slowly add 10.0 G methacryloyl chloride and 10.0 mL of acetonitrile mixed solution, moved to an oil bath at 50°C for 24 hours, cooled to room temperature...
Embodiment 3
[0047] (1) Aniline (15g, 162mmol), 160mL of water and 50mL of concentrated hydrochloric acid with a concentration of 10mol / L were mixed and stirred for 30min, then NaNO dissolved in 40mL of water was added dropwise 2 (14g). After continuing to stir for 60 min under ice-bath conditions, a phenol solution dissolved in 150 mL of 12% NaOH was added dropwise. After the dropwise addition, keep the ice bath and continue stirring for 600 min. After the reaction, filter with suction, wash the filter cake with water, and then recrystallize with a mixture of ethanol and water with a volume ratio of 1:1 to obtain a deep orange-red product 4-hydroxyazobenzene;
[0048] (2) Dissolve 1.5g N,N-dimethylaminopyridine, 40.0g triethylamine and 10.0g 4-hydroxyazobenzene in 200.0mL acetonitrile, place the mixture in an ice-water bath, and slowly add 20.0 g methacryloyl chloride and 20.0mL acetonitrile mixture, moved to an oil bath at 60°C to react for 12 hours, cooled to room temperature, added 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com