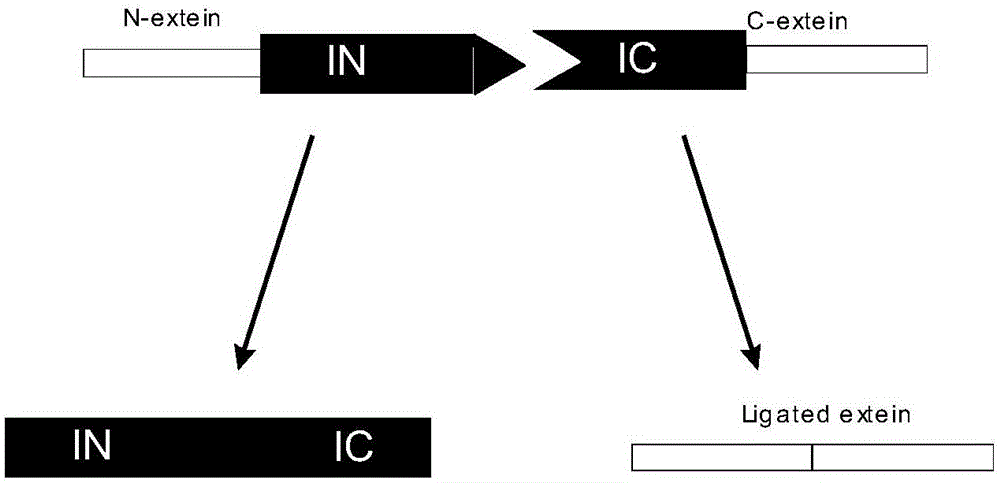
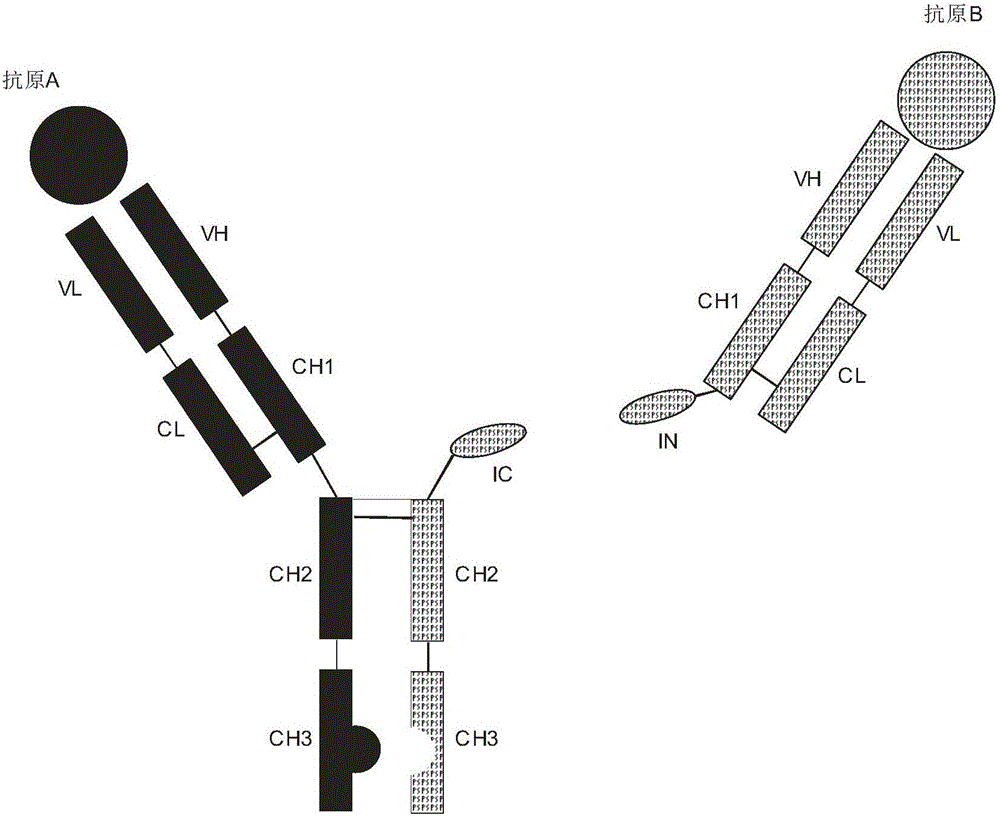
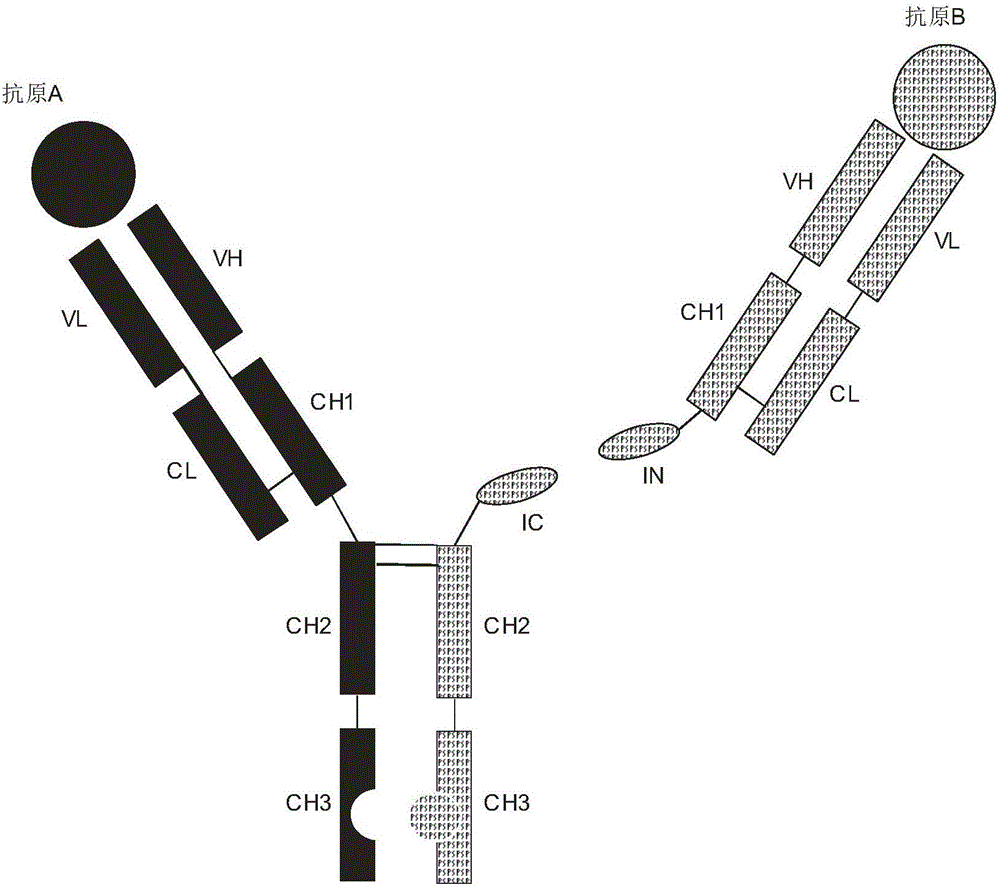
Expression and preparation methods for bivalent bispecific antibody and hybrid protein
A bispecific antibody, hybrid protein technology, applied in hybrid immunoglobulin, chemical instruments and methods, anti-animal/human immunoglobulin, etc. Heterogeneous components limit the use of drugs, etc., to avoid light chain mismatches, reduce immunogenicity, and avoid heavy chain mismatches
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0087] The specific steps of the expression and preparation method of the novel bivalent bispecific antibody hybrid protein of the present invention are as follows:
[0088] 1. Expression vector construction.
[0089] For the construction of expression vectors, general information on the nucleotide sequences of human immunoglobulin light and heavy chains is found in Kabat, E.A., et al., Sequences of Proteins of Immunological Interest, 5th edition, Public Health Service , National Institutes of Health (Public Health Service, National Institutes of Health), Bethesda, MD. (1991)) and drugbank databases. Amino acids of antibody chains are numbered and referred to according to EU numbering (Edelman, G.M., et al., Proc. , Sequences of Proteins of Immunological Interest, 5th Edition, Public Health Service, National Institutes of Health, Bethesda, MD. (1991)). Desired gene segments are prepared from oligonucleotides prepared by chemical synthesis. 600-1800bp long gene segments were...
Embodiment 1
[0108] Example 1. Construction of CD3×Her2 bispecific antibody
[0109] 1.1. Expression vector construction
[0110] For the construction of expression vectors, general information on the nucleotide sequences of human immunoglobulin light and heavy chains is found in Kabat, E.A., et al., Sequences of Proteins of Immunological Interest, 5th edition, Public Health Service , National Institutes of Health (Public Health Service, National Institutes of Health), Bethesda, MD. (1991)) and drugbank databases. Amino acids of antibody chains are numbered and referred to according to EU numbering (Edelman, G.M., et al., Proc. , Sequences of Proteins of Immunological Interest, 5th Edition, Public Health Service, National Institutes of Health, Bethesda, MD. (1991)). The CD3 antibody sequence is derived from the humanized OKT3 drug sequence, and the desired gene segment is prepared by oligonucleotides prepared by chemical synthesis. 600-1800bp long gene segments were assembled by annea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com