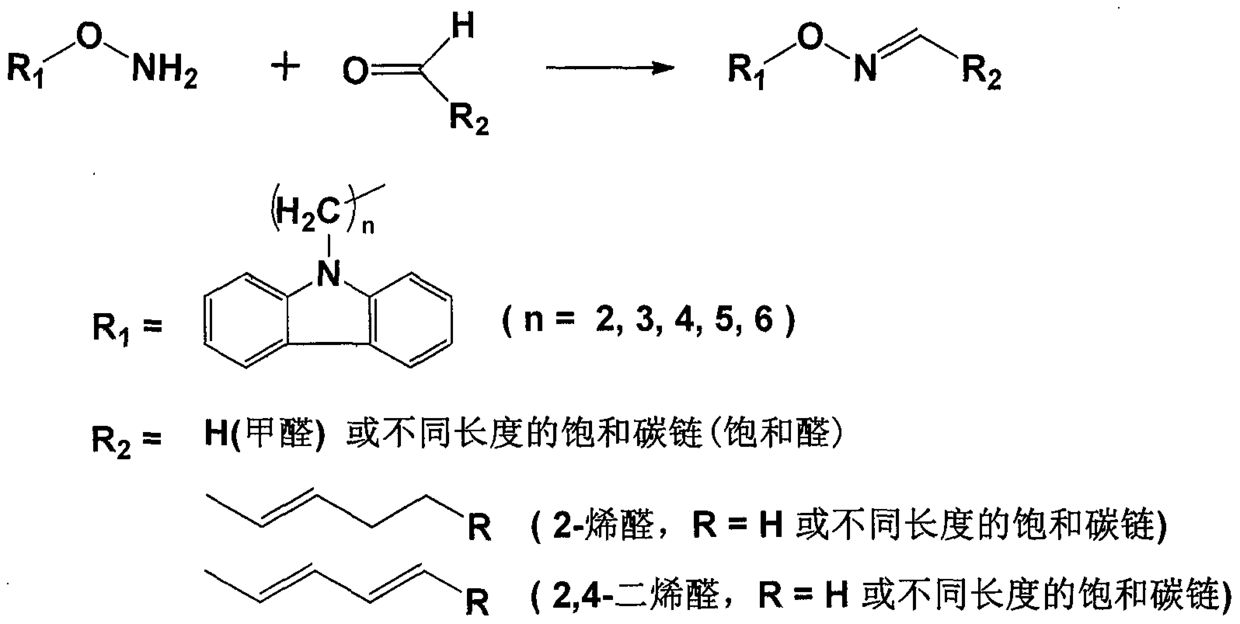
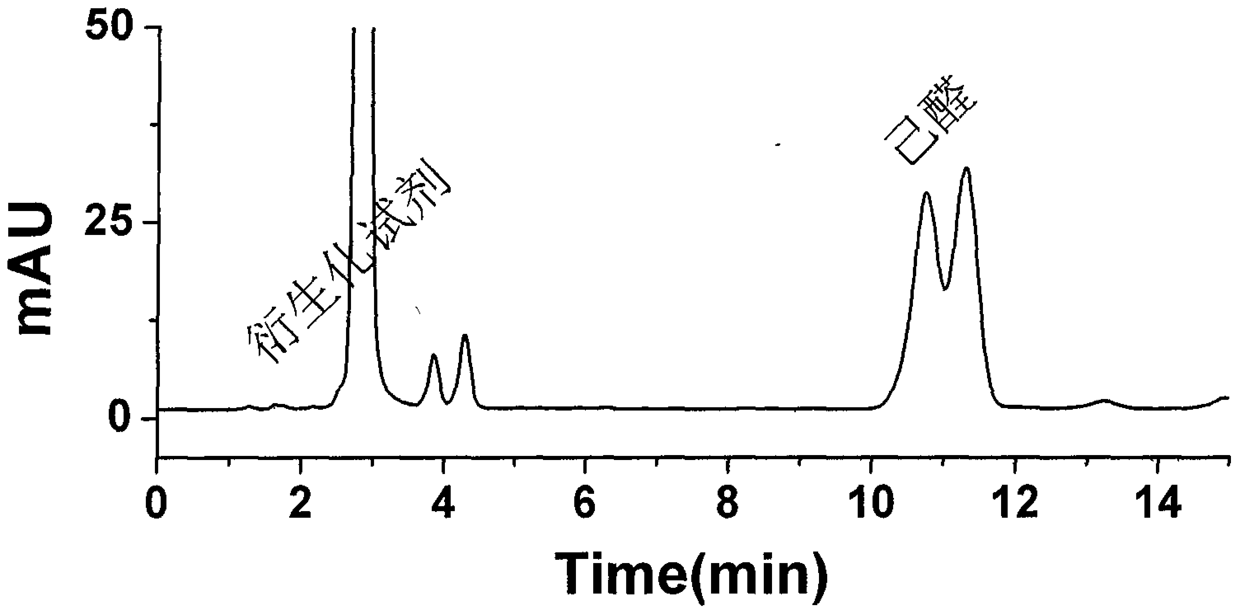
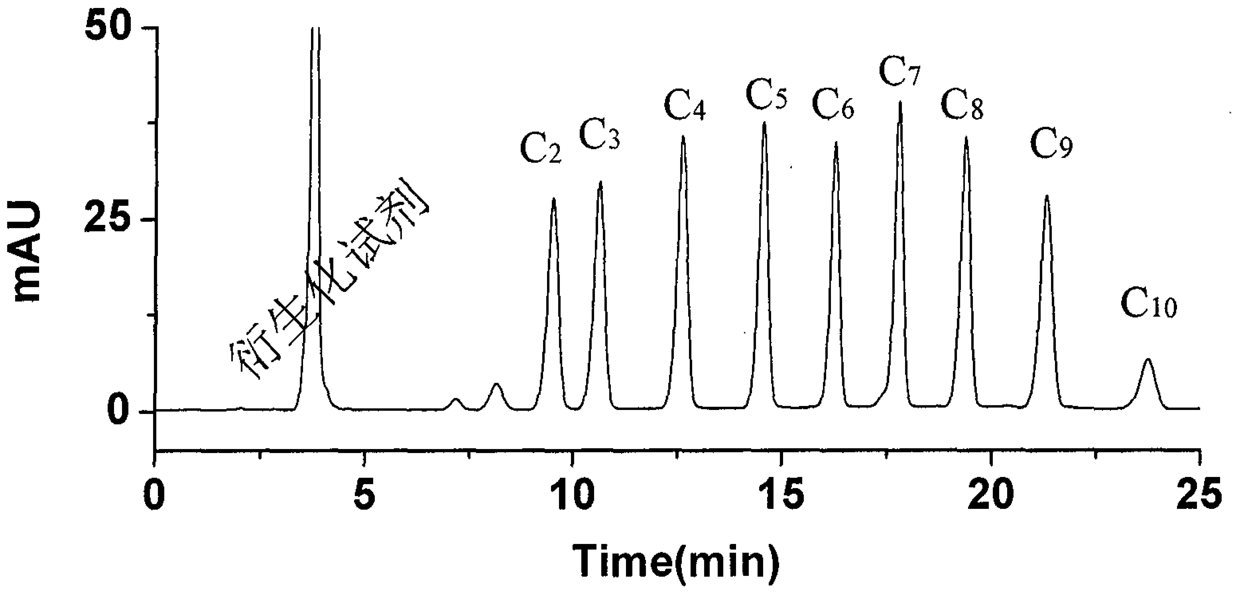
A kind of preparation and purification method of O-substituted hydroxylamine fluorescence derivatization reagent
A technology of fluorescent derivatization and purification method, which is applied in the field of preparation and purification of O-substituted hydroxylamine fluorescent derivatization reagents, can solve problems such as reduced reactivity, and achieve the effects of product stability, improved detection sensitivity and high reactivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0028] Below with 1,2-dichloroethane (simultaneously as reaction solvent) is an example to illustrate content of the present invention:
[0029] a) Add 5g of carbazole, 0.96g of tetrabutylammonium bromide and 50mL of 1,2-dichloroethane to a round bottom flask equipped with a magnetic stirrer, and add dropwise with a dropping funnel while stirring 60g of 50% KOH aqueous solution, then heated up to 90°C and reacted for 6 hours; after the reaction, the unreacted 1,2-dichloroethane was removed by rotary evaporation to obtain a brown solid, which was washed with water, filtered, and dried to obtain a light brown powder Solid, unpurified intermediate 1.
[0030] b) Add intermediate I, 5.35g of N-hydroxy-5-norbornene-2,3-diimide and 4.14g of potassium carbonate obtained in the previous step into a round-bottomed flask, and then add an appropriate amount of N,N-di Methylformamide, preferably submerged in solids, was reacted in a magnetic stirrer at 75°C for 10 hours; after the reacti...
specific Embodiment 2
[0034] Taking 1,4-dichlorobutane as an example, using tetrahydrofuran as a solvent, the content of the present invention is illustrated:
[0035] a) Add 5g carbazole, 0.96g tetrabutylammonium bromide, 10mL1,4-dichlorobutane, and 30mL tetrahydrofuran as the reaction solvent to the round-bottomed flask on the magnetic stirrer. Add 60g of 50% KOH aqueous solution to the funnel drop by drop, after the dropwise addition, heat up to 50°C and react for 2 hours; after the reaction, add water and ethyl acetate, shake well, stand to separate layers, separate the ethyl acetate layer with anhydrous Drying over sodium sulfate, rotary evaporation to remove the solvent to obtain a solid, and drying to obtain a powdery solid, which is unpurified intermediate I.
[0036] b) Add intermediate I, 5.35g N-hydroxyl-5-norbornene-2,3-diimide and 4.14g potassium carbonate obtained in the previous step into a round bottom flask, and then add an appropriate amount of N,N- Dimethylformamide, preferably ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com