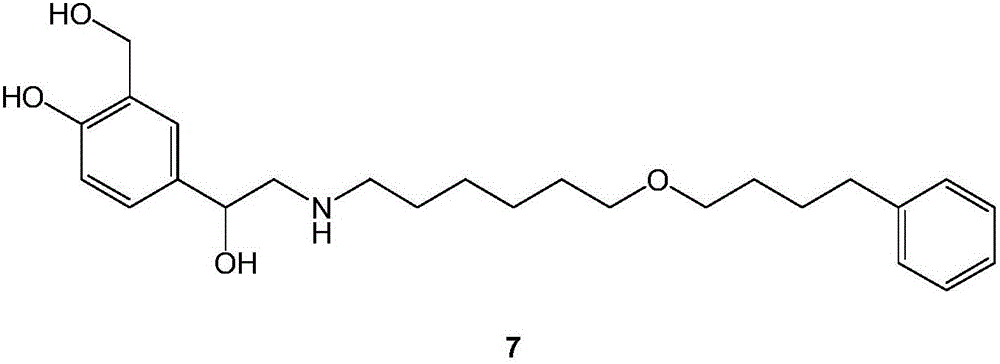
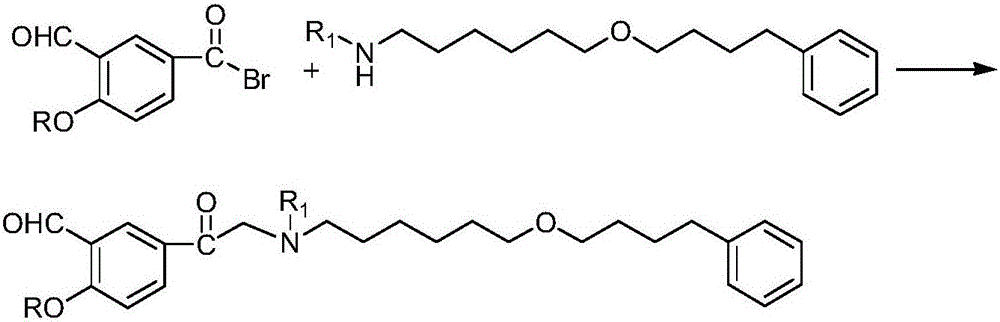
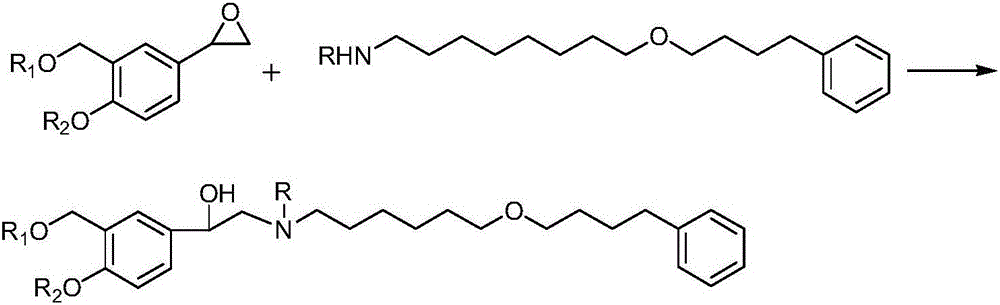
Preparation method of salmeterol base
A preparation step, benzyl technology, applied in the field of preparation of salmeterol, can solve the problems of excessive use of protective groups, difficult purification of intermediates, large loss of final products, etc., and achieve simple operation and post-treatment, and easy industrial production , low-cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Add 355g of compound 2, 2000ml of acetone and 212g of sodium bicarbonate into a 5L three-neck flask, stir to cool down, and dropwise add the acetone solution of compound 3 (438g dissolved in 1500ml of acetone) at 5°C, drop it in about 3 hours, and then keep it warm for reaction After 16 hours, the reaction solution was poured into 5L of ice water, extracted three times with 3000, 2000, and 1000ml of dichloromethane respectively, washed twice with 3000ml of saturated aqueous sodium chloride solution after merging, dried, and evaporated under reduced pressure to obtain approximately 580g oil 1.
[0045]Add 580g of oil 1 and 2000ml of ethanol from the previous step into a 5L reaction bottle, stir and cool down to about 10°C, add 380g of concentrated hydrochloric acid dropwise at -5°C, drop it in about 2 hours, and keep it warm for reaction. After 16 hours, add liquid base, adjust the pH to 8.2, filter with suction, wash, dry and evaporate the solvent under reduced pressure...
Embodiment 2
[0049] Add 355g of compound 2, 2000ml of THF and 212g of sodium bicarbonate into a 5L three-necked flask, stir and cool down, and add the THF solution of compound 3 (438g+1500mlTHF) dropwise at a temperature of 0°C to 20°C, drop it in about 3 hours, and then keep it warm for reaction 18 hours. After the TLC detection reaction was completed, the reaction solution was poured into 5L of ice water, extracted three times with 3000, 2000, and 1000ml of chloroform respectively, washed twice with 3000ml of saturated aqueous sodium chloride solution after merging, dried, and evaporated to remove the solvent under reduced pressure to obtain About 568 g of oil 1.
[0050] Add 568g of oil 1 and 2000ml of ethanol from the previous step into a 5L reaction bottle, stir and cool down to about 10°C, then add 360g of concentrated hydrochloric acid dropwise at 0°C, drop it in about 2 hours, keep it warm for about 17 hours, and check that the reaction is complete by TLC Afterwards, liquid causti...
Embodiment 3
[0054] Add 355g of compound 2, 2000ml of acetone and 212g of sodium bicarbonate into a 5L three-neck flask, stir to cool down, and dropwise add the acetone solution of compound 3 (438g dissolved in 1500ml of acetone) at 10°C, drop it in about 3 hours, and then keep it warm for reaction After 16 hours, the reaction solution was poured into 5L of ice water, extracted three times with 3000, 2000, and 1000ml of dichloromethane respectively, washed twice with 3000ml of saturated aqueous sodium chloride solution after merging, dried, and evaporated under reduced pressure to obtain approximately 580g oil 2.
[0055] Add 580g of oily substance 2 and 2000ml of ethanol from the previous step into a 5L reaction bottle, stir and cool down to about 10°C, add 380g of concentrated hydrochloric acid dropwise at 10°C, drop it in about 2 hours, keep it warm for reaction, and add liquid caustic soda dropwise after 16 hours , adjust the pH to 8.5, filter with suction, wash, dry and distill off th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com