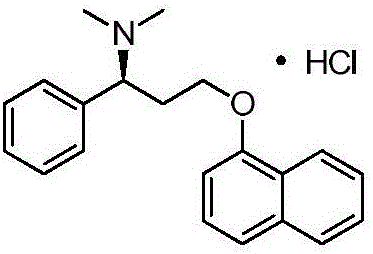
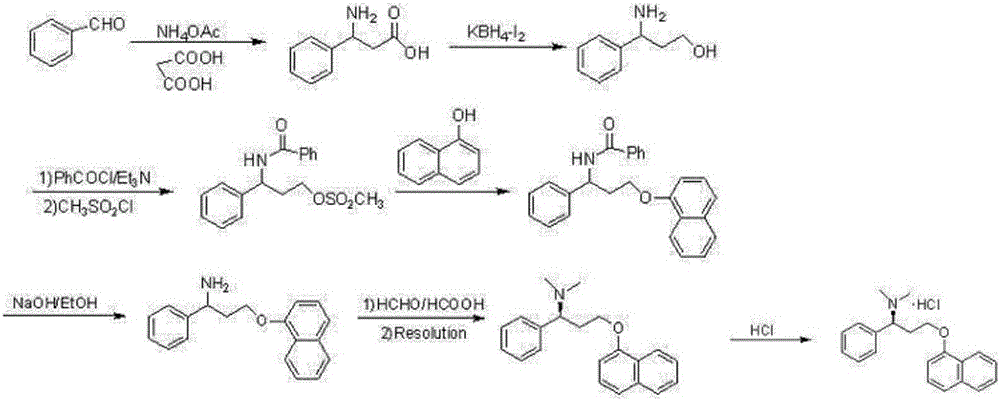
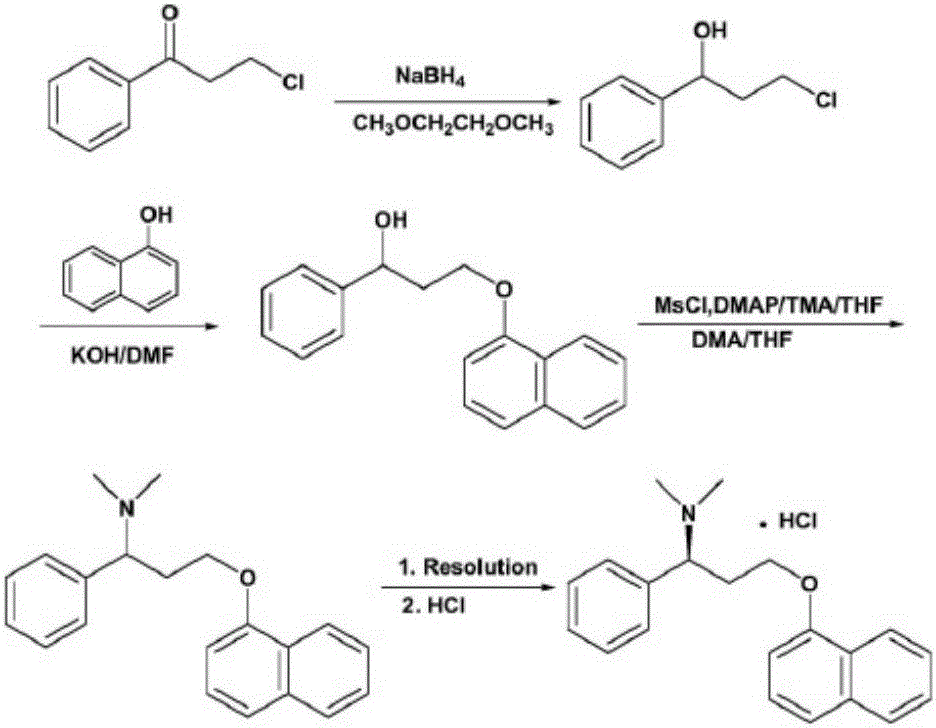
Preparation method of dapoxetine hydrochloride
A dapoxetine hydrochloride and compound technology, which is applied in the field of preparation of dapoxetine hydrochloride, can solve the problems of low yield and low optical purity, and achieve simple post-processing, chemical purity and optical purity High, optically pure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Dissolve 16.85g of compound of formula VI and 14.51g of compound of formula V in 150mL of dry tetrahydrofuran, add 5.60g of solid KOH, heat to 60°C for reaction, monitor by TLC, after the compound of formula VI is completely reacted, cool to room temperature, add 20g of water CuSO 4 And 14.52g (S)-tert-butylsulfinamide, under stirring, add 27.36g condensing agent Ti(OEt) 4 , reflux reaction, after the reaction, pour the reaction solution into ethyl acetate and brine, separate the organic layer, extract the water layer with ethyl acetate several times, combine the organic layers, concentrate to oil, add isopropyl ether, stir 34.5 g of the intermediate of formula IV was precipitated as a pale yellow solid, with a yield of 91.0% and a purity of 95% (HPLC area normalization method).
[0040] MS(ES+) m / z: 402[M+Na] + . 1 H-NMR (CDCl 3 )δ: 1.37(s,9H); 3.71-4.06(m,2H); 4.46-4.59(m,2H); 6.83(d,1H); 7.26-7.52(m,8H); 7.77(m,1H) ; 8.12(m,1H).
Embodiment 2
[0042] Dissolve 18.8g of the compound of formula IV in 150mL of anhydrous THF, lower the temperature to below 0°C, and add the reducing agent BH in batches 3Tetrahydrofuran complex (50mL, 1eq), after the addition is complete, continue to stir the reaction below 0°C, and the reaction is completed for 3h; add HCl ethanol solution (3eq) to the reaction solution at room temperature, stir for 0.5-2h, concentrate under reduced pressure, add Stir with isopropyl ether, filter, dissolve the filter cake in water, slowly add 10% NaOH aqueous solution dropwise, adjust the pH to be greater than or equal to 7, then extract with ethyl acetate (90mL×2), dry, and concentrate to obtain intermediate 12.6 of formula III g, yield 91.9%, purity 98% (HPLC area normalization method), 99% ee.
[0043] MS(ES+) m / z: 278[M+H] + . 1 H-NMR (CDCl 3 )δ: 2.33(m,2H); 4.10(m,1H); 4.21(m,1H); 4.33(m,1H); 6.73(d,1H); 7.26-7.49(m,8H); ,1H); 8.23(m,1H).
Embodiment 3
[0045] Dissolve 10 g of the compound of formula III in 80 mL of CH 2 Cl 2 , add 12mL triethylamine, lower the temperature to below 0°C, put 6mL CH 3 I was dissolved in 20 mL CH 2 Cl 2 , and then drop it slowly, drop it, react at room temperature for 5h, after the reaction, add Na 2 SO 3 The aqueous solution was stirred and allowed to stand, and the organic layer was separated, dried, and evaporated to dryness to obtain 9.8 g of the compound of formula II, namely dapoxetine, with a yield of 89.1% and a purity of 94% (HPLC area normalization method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com