Purified 1-hydroxy-4-arylaminoanthraquinone product and preparation method thereof
An arylaminoanthracene and dihydroxyanthraquinone technology is applied in the field of purified 1-hydroxy-4-arylaminoanthraquinone products and their preparation, and can solve the problems of high price, uneconomical single condensed dyes and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
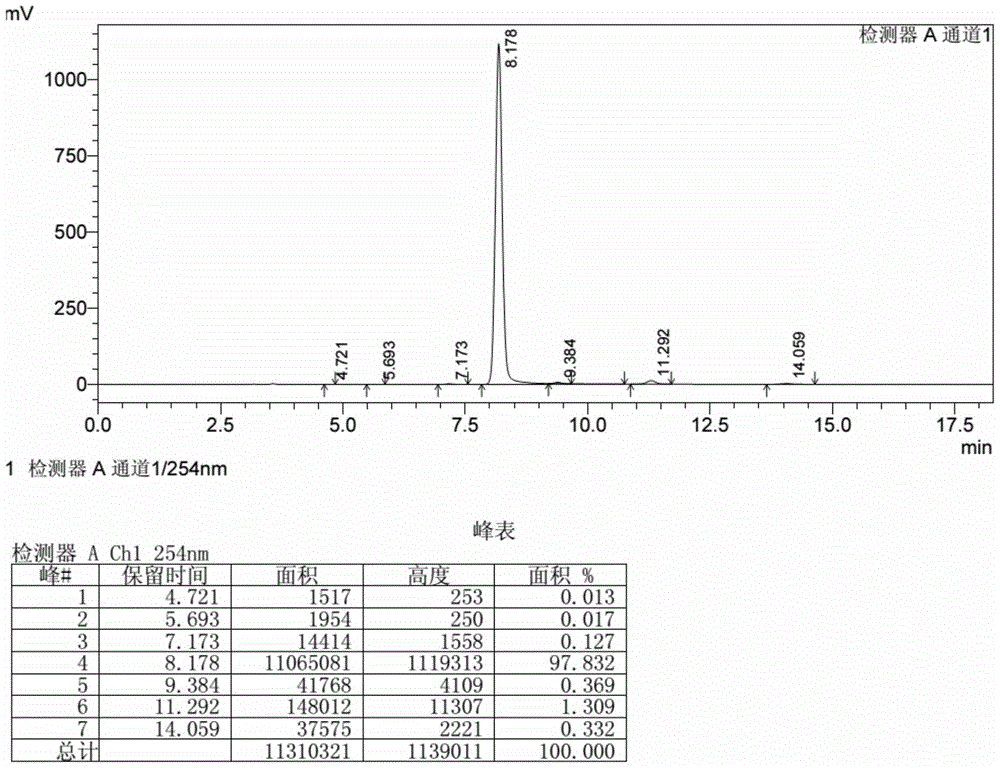
[0027] In a 1.0L autoclave equipped with special stirring, add 520ml of water, 3.2 grams of sodium dodecylbenzenesulfonate, 44 grams of aniline, 80 grams of quinizarin, 28 grams of quinizarin leuco, and the mixture is heated under air isolation to 110°C, keep it for 15 hours, cool down to 60°C, discharge and filter, wash the filter cake with 80°C hot water, and dry at 100°C to obtain 133.3 grams of purple powdery product. The above product was added to 355 grams of 60% DMF-containing aqueous solution, heated and refluxed for 2 hours under stirring, then cooled to room temperature, filtered, washed with hot water, and dried at 105° C. to obtain 126.4 grams of reddish-purple crystalline powder. analyzed by liquid chromatography (see figure 1 ), the main product is 1-hydroxy-4-anilinoanthraquinone content of 97.8%, impurity 1,4-dianilino anthraquinone content of 1.3%, 1,4-dihydroxy anthraquinone content of 0.13%, and other unknown impurities. 0.37% max.
Embodiment 2
[0029] The procedure described in Example 1 was repeated, but using 3.8 g of sodium cetyl sulphate, to give the same product.
Embodiment 3
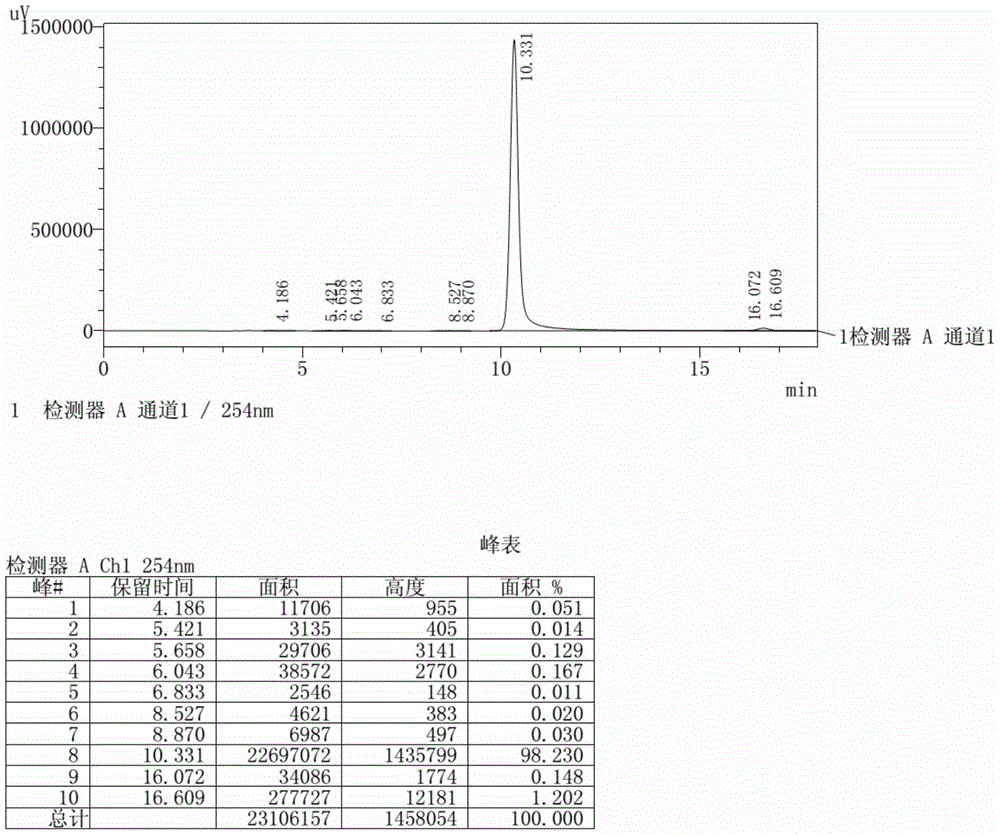
[0031]In a 1.0L autoclave equipped with special stirring, add 600ml of water, 2.6g of diffusing agent N and 49.8g of p-toluidine, 80g of quinizarin, 28g of quinizarin leuco, and the mixture is heated to 110°C under the isolation of air , kept for 18 hours, cooled to 60°C, filtered the discharge, washed the filter cake with hot water, and dried at 100°C to obtain 140.6 grams of dark purple powdery product. The above product was added to 350 grams of an aqueous solution containing 80% DMF, heated and refluxed for 2 hours under stirring, then cooled to room temperature, filtered, washed with hot water, and dried at 105°C to obtain 128.6 grams of blue-purple crystalline powder, which was analyzed by liquid chromatography (see figure 2 ), the main product is 1-hydroxy-4-p-toluidino anthraquinone with a content of 98.2%, the impurity 1,4-di-p-toluidino anthraquinone with a content of 1.20%, 1,4-dihydroxy anthraquinone with a content of 0.17%, and others unknown The sum of impuriti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com