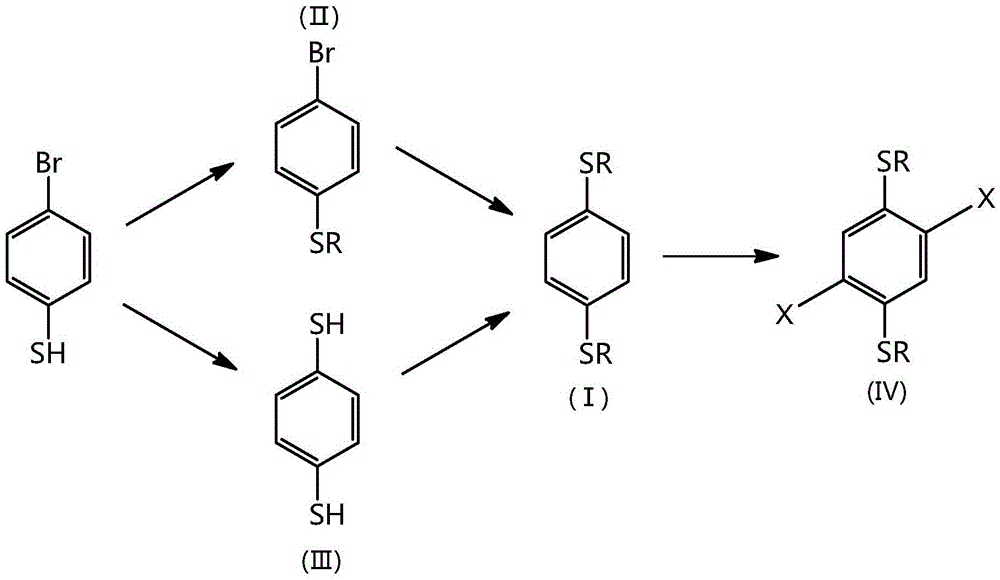
Synthetic method for 1,4-dithioalkyl benzene and halide thereof
A technology of disulfide benzene and benzene dithiol, which is applied in the preparation of hydrogenated polysulfides/polysulfides, mercaptans, and sulfides, and can solve the problem of high production costs and the flammability and explosion of tert-butyllithium , many reaction steps, etc., to achieve the effect of large-scale industrial production, good safety and controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Feed ratio: 4-bromothiophenol: potassium carbonate: 1-bromo-2-hexyldecane=1:1.5:1 (molar ratio), N,N-dimethylformamide is used as an organic solvent, and its consumption is 4 -5 times that of bromothiophenol.
[0014] Add 10 g (52.89 mmol) of 4-bromothiophenol, 100 mL of N, N-dimethylformamide, and 10.94 g (79.33 mmol) of anhydrous potassium carbonate into a 250 mL three-necked flask equipped with a magnetic stirrer and a reflux condenser. Under argon protection, stirred at room temperature for 30 minutes, 1-bromo-2-hexyldecane 16.2mL (52.89mmol) was added dropwise into the reaction system with a constant pressure dropping funnel, and the reaction temperature was gradually raised to 85°C. React for 24 hours. After the reaction was completed, cool to room temperature, pour the reaction solution into 150 mL of ice water, stir for 10 minutes, then extract petroleum ether as the extractant 3 times, 100 mL each time, combine the organic phases, and wash the organic phases w...
Embodiment 2
[0020] Feed ratio: 4-bromothiophenol: n-butyllithium: sulfur powder=1:2:1 (molar ratio), tetrahydrofuran is used as an organic solvent, and its dosage is 5 times that of 4-bromothiophenol.
[0021] In a 50mL three-necked flask equipped with a magnetic stirrer, 1.037g (5.48mmol) of 4-bromothiophenol was fully dissolved in 5mL of anhydrous tetrahydrofuran, cooled to -78°C under the protection of argon, and then 4.4mL (2.5 mol / L, 10.96mmol) n-butyllithium solution, after dripping, continue to react at -78°C for 2 hours, add sulfur powder 0.175g (5.48mmol) at low temperature, continue to react for 3 hours, add 10 % hydrochloric acid solution 10mL, stirred and reacted for 1 hour, after the reaction was completed, dichloromethane was used as the extractant, extracted 3 times, 15mL each time, the organic phase was combined, and the organic phase was washed 3 times with distilled water, 20mL each time. The organic phase was dried with anhydrous sodium sulfate, filtered with suction, a...
Embodiment 3
[0028] 2,5-dibromo-1,4-bis(2-hexyldecylthio)benzene preparation ratio is: 1,4-bis(2-hexyldecylthio)benzene: liquid bromine=1:2.5 (molar ratio), dichloromethane is used as an organic solvent, and its consumption is 5 times of 1,4-bis(2-hexyldecylthio)benzene.
[0029] Add 2.4g (4.06mmol) 1,4-(2-hexyldecylthio)benzene and 25mL dichloromethane into a 100mL two-necked flask equipped with a magnetic stirrer and a constant pressure dropping funnel, stir well, and then add iodine Elemental substance 0.026g (0.1mmol), and liquid bromine 0.52mL (10.15mmol) was slowly added dropwise to the reaction solution with a constant pressure dropping funnel under ice bath. After the reaction was completed, the reaction solution was poured into 50 mL of sodium thiosulfate aqueous solution, stirred for 10 minutes, and then extracted three times with petroleum ether, 25 mL each time, the organic phases were combined, and the organic phase was washed with water 3 times, 30 mL each time. The organic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com