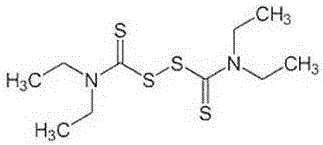
Anti-cancer transdermic absorption preparation
An anti-cancer and preparation technology, applied in the field of percutaneous absorption preparations, to achieve the effects of easy acceptance, high controllability and good absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] (1) Formulation 1-3
[0053] Table 1 Formulations 1-3
[0054]
[0055] Remarks: For this recipe and the following recipes, every 1 serving is equivalent to 1%, every 10 servings is equivalent to 10%, and so on.
[0056] (2) Method
[0057] Preparation of oil phase: Take the thickener, emulsifier (part) and matrix in the prescribed amount and add them to the beaker in turn, shake well, heat in a water bath to 80°C, and keep warm. Separately take the formula amount of transdermal accelerator and preservative, put it in the Erlenmeyer flask, shake it with the formula amount of absolute ethanol, dissolve it, add the formula amount of the main drug, heat and shake it in a water bath at 80°C, then add it to the above-mentioned 1 beaker In the process, use a constant speed high-speed dispersing homogenizer, 7kr / min, and homogenize for 2 minutes.
[0058] Water phase preparation: Put the formulated amount of emulsifier (the remaining part), humectant, inclusion agent and...
Embodiment 2
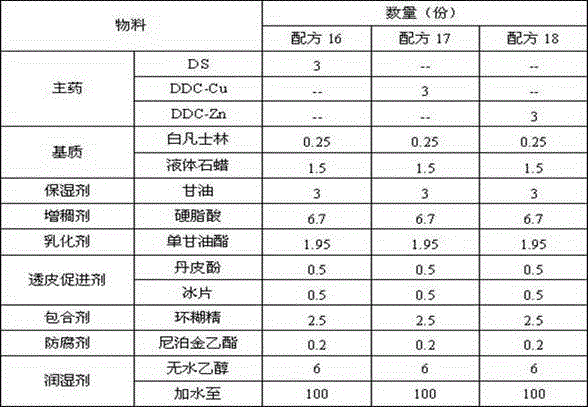
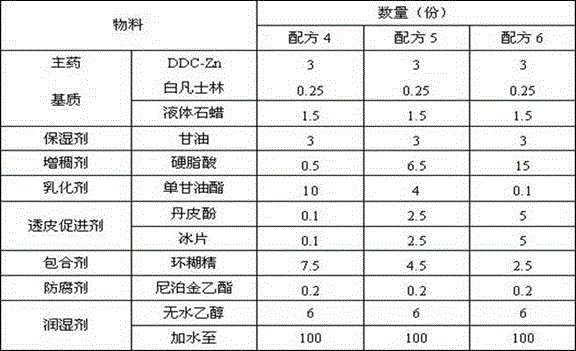
[0061] (1) Formulation 4-6
[0062] Table 2 Formulations 4-6
[0063]
[0064] (2) Method
[0065] The preparation method is the same as that of formula 1-3.
Embodiment 3
[0067] (1) Recipe 7-9
[0068] Table 3 Formulations 7-9
[0069]
[0070] (2) Method
[0071] The preparation method is the same as that of formula 1-3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com