A kind of triblock polymer, its preparation method and application
A technology of polymer, polymer glue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: Preparation of polyethylene glycol-aminated polyglycidyl methacrylate-polydiisopropylaminoethyl methacrylate polymer
[0053] Take 1.0 g of polyethylene glycol 5000 macromolecular initiator modified by acid bromide and dissolve it in 1.44 ml N,N-dimethylacetamide, stir and mix well, add glycidyl methacrylate 0.525 ml, and initiate with the above macromolecular Equimolar cuprous chloride catalyst and pentamethyldiethylenetriamine were reacted at 40°C for 15 hours to obtain a methoxy-terminated polyethylene glycol-polyglycidyl methacrylate diblock copolymer.
[0054] Take 0.2 g of the above-prepared methoxy-terminated polyethylene glycol-polyglycidyl methacrylate diblock copolymer and dissolve it in 0.3 ml of N,N-dimethylacetamide, stir and mix, and then add formaldehyde 0.4 ml of diisopropylaminoethyl acrylate monomer, cuprous chloride catalyst and pentamethyldiethylenetriamine equimolar to the above two-block copolymer, reacted at 40°C for 48 hours. Vacuu...
Embodiment 2
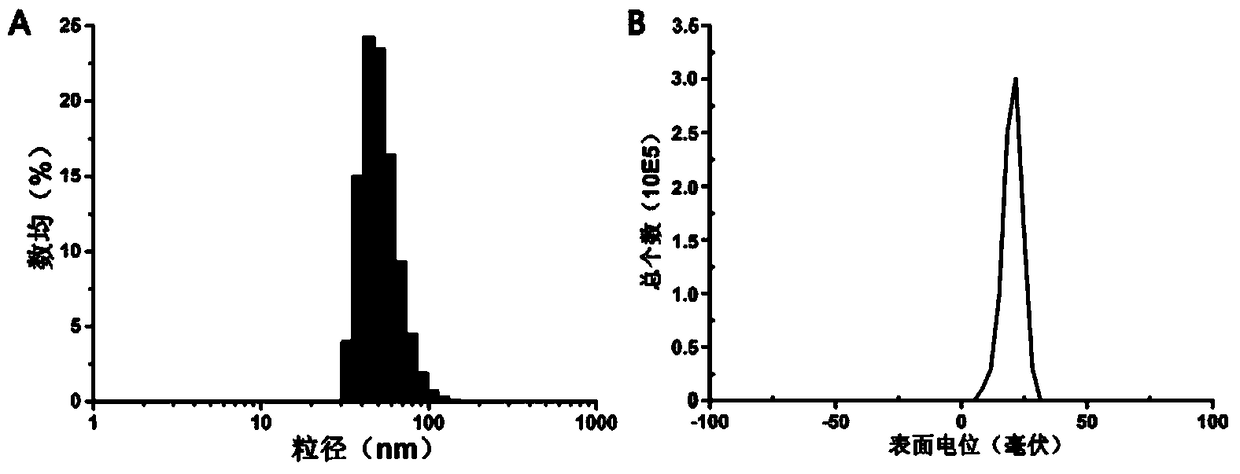
[0056] Embodiment 2. Preparation of polymer micelles
[0057]Take 25 mg polyethylene glycol-aminated polyglycidyl methacrylate-polydiisopropylaminoethyl methacrylate triblock polymer (wherein the polymerization degree of polyethylene glycol is 113, and the aminated polymethylmethacrylate The degree of polymerization of glycidyl acrylate is 50, and the degree of polymerization of polydiisopropylaminoethyl methacrylate is 60) dissolved in 0.2ml N,N'-dimethylacetamide, and added dropwise under the condition of ultrasonic (200mW) into 1.8ml of deionized water, vortexed (1000rpm) for 1 minute, and dialyzed in deionized water for 24 hours with a dialysis bag with a molecular weight cut-off of 3500 Daltons to obtain polymer micelles.
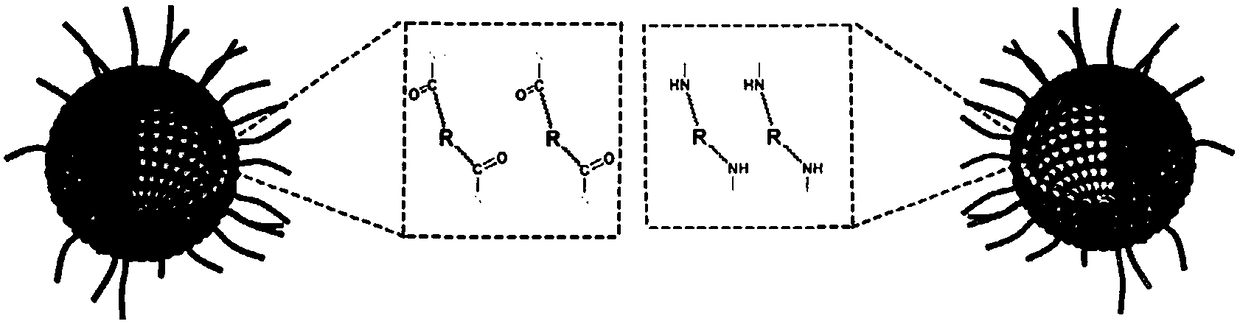
Embodiment 3
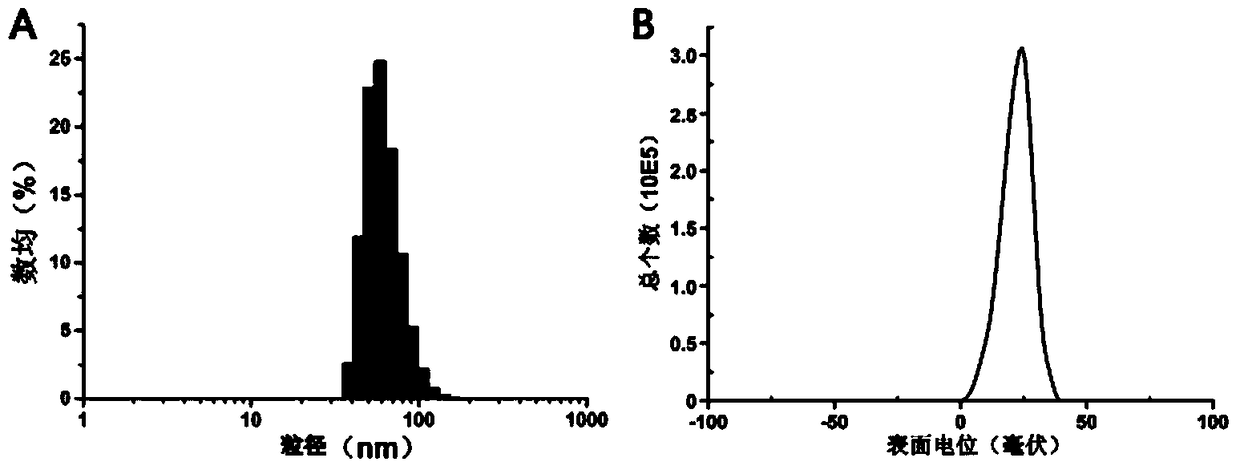
[0058] Example 3. Preparation of chemically crosslinked polymer micelles
[0059] Get double-terminal carboxylated cisplatin (compound of formula 3) 16mg, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride 18mg and 1-hydroxybenzotriazole 9.6mg, dissolve In 0.2ml dimethyl sulfoxide. After the activation reaction at room temperature for 2 hours, it was added dropwise to the polymer micelles prepared in Example 2 under ultrasonic conditions (200 mW), and the reaction was continued at room temperature for 12 hours. Carboxylated cisplatin-crosslinked polymer micelles were obtained after dialysis with a dialysis bag with a molecular weight cut-off of 3500 Daltons in deionized water for 24 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| hydrodynamic diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com