1-phenyl-1-(cyclohexyl methyl) base ethane isomer of heat conduction fluid and synthesis method of isomer
A technology of cyclohexyltoluene and heat transfer fluid, which is applied in the field of heat transfer fluid technology and chemical synthesis, which can solve the problems of high synthesis reaction temperature and low use of ultra-high temperature heat transfer oil, and achieve the effects of high flash point, low vapor pressure and low freezing point
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
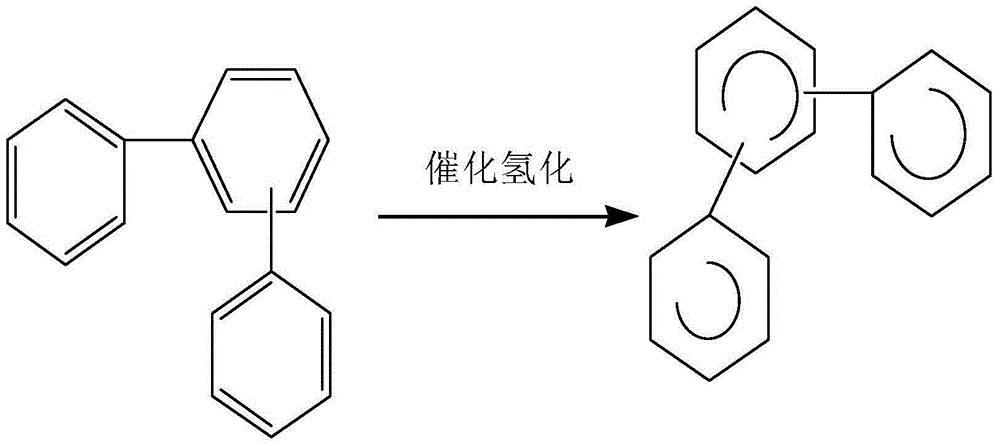
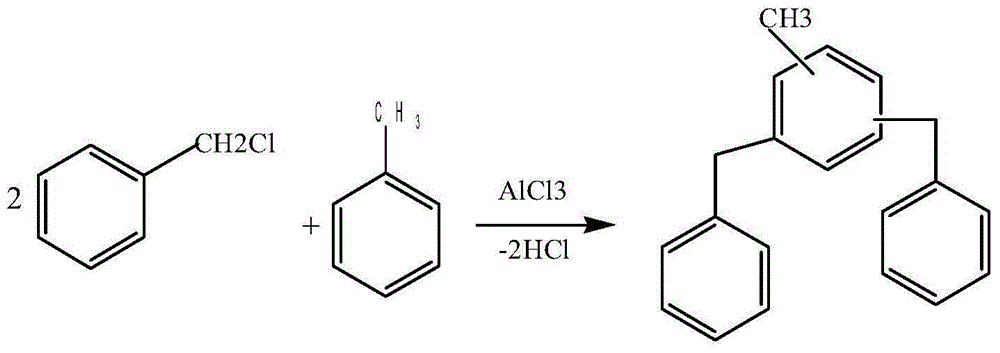
Image
Examples
Embodiment 1
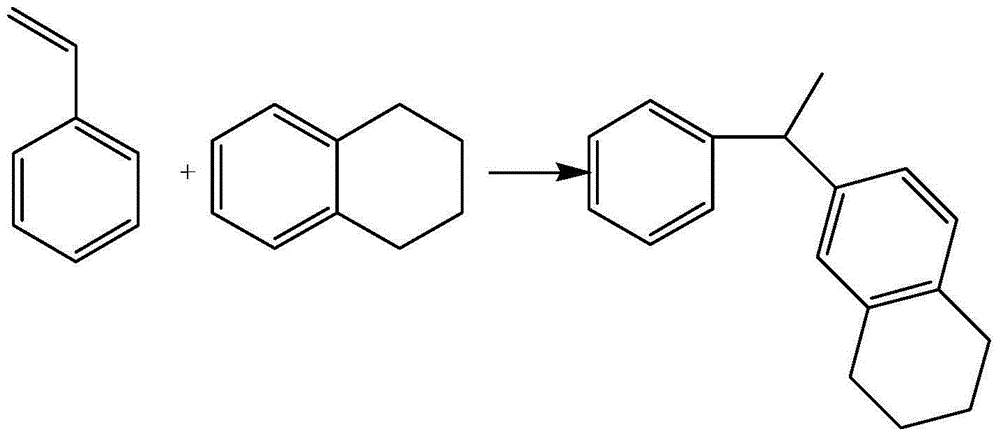
[0022] A heat-conducting fluid 1-phenyl-1-(cyclohexyltoluene) ethane isomer, the structural formula is as follows:
[0023]
[0024] The specific synthesis method is that cyclohexyltoluene and styrene are catalyzed by a fluorinated protonic acid and a solid Lewis acid complex at a temperature of 35-55°C, and the F-C reaction alkylation occurs, wherein cyclohexyltoluene and The molar ratio of styrene is 5-10:1. The fluorinated protonic acid is trifluoroacetic acid, the solid Lewis acid complex is aluminum trichloride, the amount of aluminum trichloride is 0.5-1.5% of the weight of cyclohexyltoluene, and the mass ratio of trifluoroacetic acid to aluminum trichloride is 1 : 50-100. The reaction formula is as follows:
[0025]
[0026] After the reaction is finished, after separating and removing the fluorinated protonic acid and the solid Lewis acid, rectifying and recovering excess cyclohexyl toluene, and then rectifying under negative pressure, the obtained product is o...
Embodiment 2
[0028] Specifically, the synthesis steps of the present invention are as follows:
[0029] 1) Cyclohexyltoluene is dropped into the reactor;
[0030] 2) adding anhydrous aluminum trichloride accounting for 0.5-1.5% of the mass of cyclohexyltoluene and trifluoroacetic acid accounting for 1-2% of the mass of anhydrous aluminum trichloride;
[0031] 3) Control the temperature from 25 to 35°C, maintain the temperature, and gradually add styrene dropwise within 1-2 hours under stirring;
[0032] 4) After feeding, keep warm for 0.5-1 hour to make the reaction complete;
[0033] 5) Add zinc oxide of the same weight as aluminum trichloride and a small amount of water to decompose aluminum trioxide and remove the generated hydrochloric acid, raise the temperature to 80-120°C, keep warm and stir for 1-2 hours, stand still, filter to obtain the filtrate ;
[0034] 6) Add an appropriate amount of activated clay to the filtrate obtained in step 5), raise the temperature to 50-120°C, and...
Embodiment 3
[0037] 1) Add 595g cyclohexyltoluene (3.5moles), 3.6g anhydrous aluminum trichloride, 75mg trifluoroacetic acid in the reactor;
[0038] 2) Heat to 25-35°C, add 54g (0.5mole) styrene dropwise with stirring within 2 hours;
[0039] 3) After the dropwise addition, keep the temperature and react for 0.5 hours; then add 4g of zinc oxide and 1g of water, raise the temperature to 82°C and react for 1.5 hours.
[0040] 4) be down to room temperature, filter, obtain filtrate;
[0041] 5) Mix the filtrate obtained in step 4) with 5 g of activated clay, heat to 100 ° C, stir for 15 minutes, reach neutral, drop to room temperature, and filter;
[0042] 6) filtrate negative pressure fractionation, first obtain unnecessary cyclohexyl toluene;
[0043] 7) Further rectification under reduced pressure to obtain 1-phenyl-1-(cyclohexyltolyl)ylethane isomer with a content of 99.5% and a yield of 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com