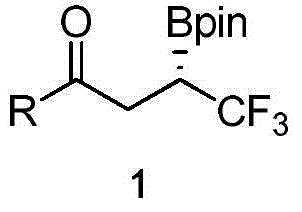
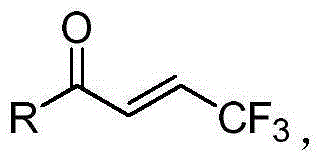
Method for synthesizing chiral boron compound containing trifluoromethyl and compound
A technology of trifluoromethyl group and synthesis method is applied in the field of synthesis of trifluoromethyl group-containing chiral boron compounds, and achieves the effects of easy preparation, easy availability of raw materials and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
[0035] Under nitrogen protection, copper iodide CuI (1.9mg, 0.01mmol), (R,S)-Josiphos (9.6mg, 0.015mmol), potassium phosphate K 3 PO 4 (4.2mg, 0.02mmol), pivalyl alcohol t-AmOH (1.0mL), after stirring at room temperature for 30 minutes, add bis-pinacolyl diborane 3 (55.9mg, 0.22mmol) and pivalyl alcohol t-AmOH (0.5 mL), stirred at room temperature for 10 minutes. Then (E)-4,4,4-trifluoro-1-p-tolyl-2-en-1-one 2a and pivalyl alcohol t-AmOH (0.5 mL) were added, and stirred at 60° C. for 20 hours. After cooling to room temperature, filter through diatomaceous earth, wash the filter cake with ethyl acetate (20 mL), and concentrate the filtrate under reduced pressure. After separation by silica gel column chromatography (eluent: petroleum ether (60-90°C) / ethyl acetate, v / v=60:1), the white solid product 1a (43mg, yield 63%, ee value 93% ). The target product was confirmed by NMR and high-resolution mass spectrometry, and the ee value was determined by chiral high-per...
Embodiment 2
[0037] The reaction steps and operation are the same as in Example 1, except that the copper salt is cuprous bromide. The reaction was stopped, and the target product 1a (39 mg, yield 58%, ee value 93%) was obtained after post-processing. It shows that cuprous bromide can also be used as a catalyst for the reaction, but it is not the best catalyst.
Embodiment 3
[0039] The reaction steps and operation are the same as in Example 1, and the difference from Example 1 is that the copper salt is cuprous chloride. The reaction was stopped, and the target product 1a (6 mg, yield 9%) was obtained after post-processing. It shows that cuprous chloride can also be used as a catalyst for the reaction, but it is not the best catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com