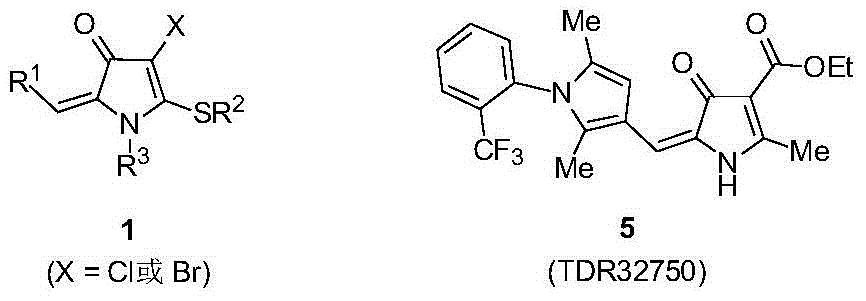
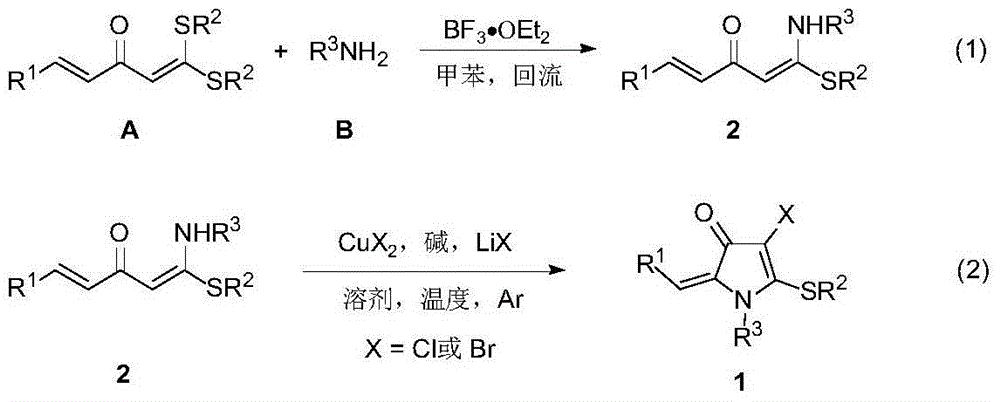
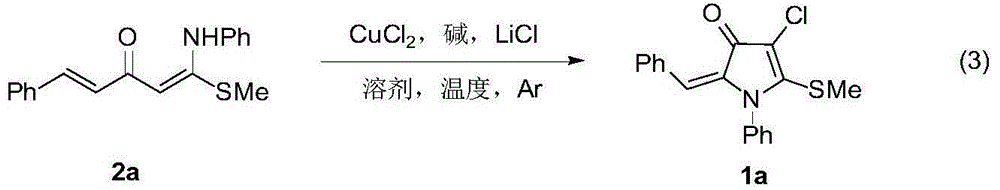
4-chloro(bromo)-5-alkylthio-3-pyrrolidone derivative and synthesis thereof
A technology of alkylthio and pyrrolidone, which is applied in the field of 4-chloro(bromo)-5-alkylthio-3-pyrrolone derivatives and synthesis thereof, and achieves the effects of low cost, easy industrial production and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] In the glove box, weigh 1-thiomethyl-1-anilino-5-phenyl-1,4-pentadien-3-one 2a (0.5mmol), copper chloride (2.0mmol), carbonic acid Potassium (2.0mmol), lithium chloride (1.5mmol) in a 25mL Schlenk reaction flask, under argon, add N,N-dimethylformamide (DMF) solvent 5mL, stir at room temperature for 2 minutes, put in 100 °C in an oil bath for 12 hours. After the reaction, the mixture was cooled to room temperature, filtered with diatomaceous earth, extracted with ethyl acetate and 10% ammonia water in mass concentration, collected the organic phase, dried over anhydrous magnesium sulfate, filtered, removed volatile components under reduced pressure, and then used a silica gel column to Chromatographic separation (eluent: petroleum ether (60-90°C) / ethyl acetate, v / v=20:1) gave the target product 1a as a red solid (115 mg, yield 70%). The target product was confirmed by NMR and high-resolution mass spectrometry.
Embodiment 2
[0032]
[0033] In the glove box, weigh 1-thiomethyl-1-p-methoxyanilino-5-phenyl-1,4-pentadien-3-one 2b (0.5 mmol), copper chloride (1.5 mmol), potassium phosphate (1.5mmol), and lithium chloride (1.5mmol) in a 25mL Schlenk reaction flask, under argon, add 5mL of DMF solvent, stir at room temperature for 2 minutes, and put it into an oil bath at 120°C for reaction 6 Hour. After the reaction, the mixture was cooled to room temperature, filtered with celite, extracted with ethyl acetate and 10% ammonia water, the organic phase was collected, dried over anhydrous magnesium sulfate, filtered, and the volatile components were removed under reduced pressure, followed by silica gel column chromatography Separation (eluent: petroleum ether (60-90°C) / ethyl acetate, v / v=20:1) gave the target product 1b (166 mg, yield 93%) as a red solid. The target product was confirmed by NMR and high-resolution mass spectrometry.
Embodiment 3
[0035]
[0036]In the glove box, successively weigh 1-thiomethyl-1-m-fluoroanilino-5-phenyl-1,4-pentadien-3-one 2c (0.5mmol), copper chloride (2.0mmol) , potassium phosphate (2.0mmol), and lithium chloride (1.5mmol) were placed in a 25mL Schlenk reaction flask, under argon, 5mL of DMF solvent was added, stirred at room temperature for 2 minutes, and placed in an oil bath at 80°C for 2 hours. After the reaction, the mixture was cooled to room temperature, filtered with celite, extracted with ethyl acetate and 10% ammonia water, the organic phase was collected, dried over anhydrous magnesium sulfate, filtered, and the volatile components were removed under reduced pressure, followed by silica gel column chromatography Separation (eluent: petroleum ether (60-90°C) / ethyl acetate, v / v=20:1) gave the target product 1c (159 mg, yield 92%) as a red solid. The target product was confirmed by NMR and high-resolution mass spectrometry.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com