Lithium ion battery positive electrode material with ultrahigh energy density and preparation method of lithium ion battery positive electrode material with ultrahigh energy density
A lithium-ion battery, cathode material technology, applied in battery electrodes, circuits, electrical components, etc., can solve problems such as low capacity and poor electrochemical performance, and achieve the effects of improving discharge specific capacity, inhibiting structure collapse, and good cycle performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
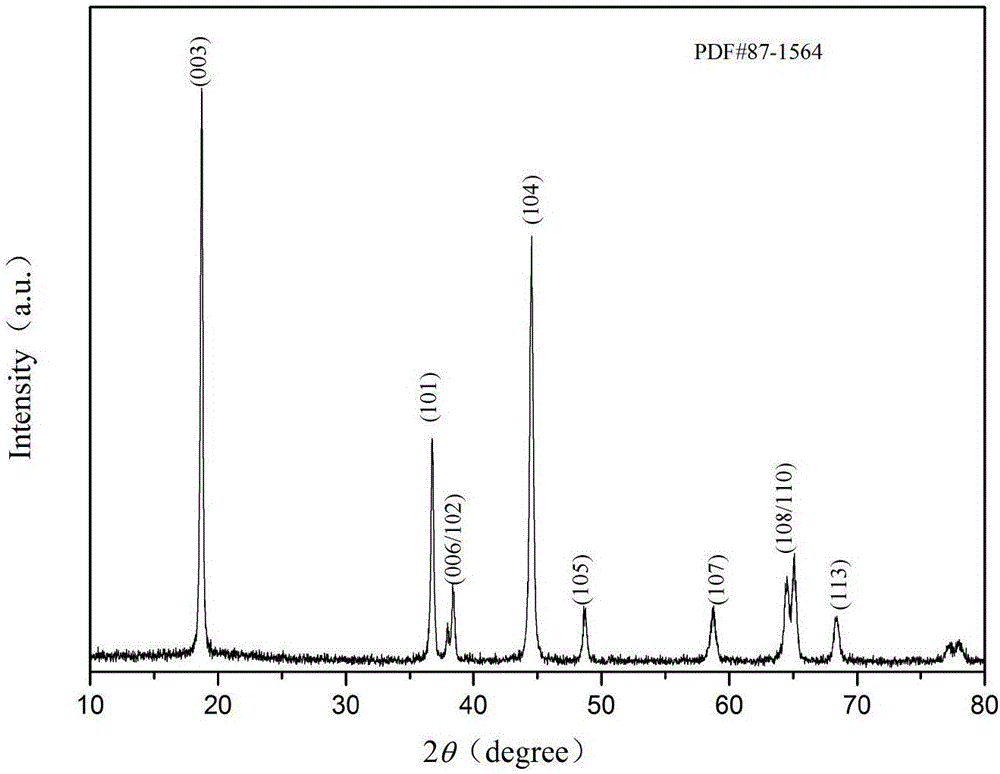
Image
Examples
Embodiment 1
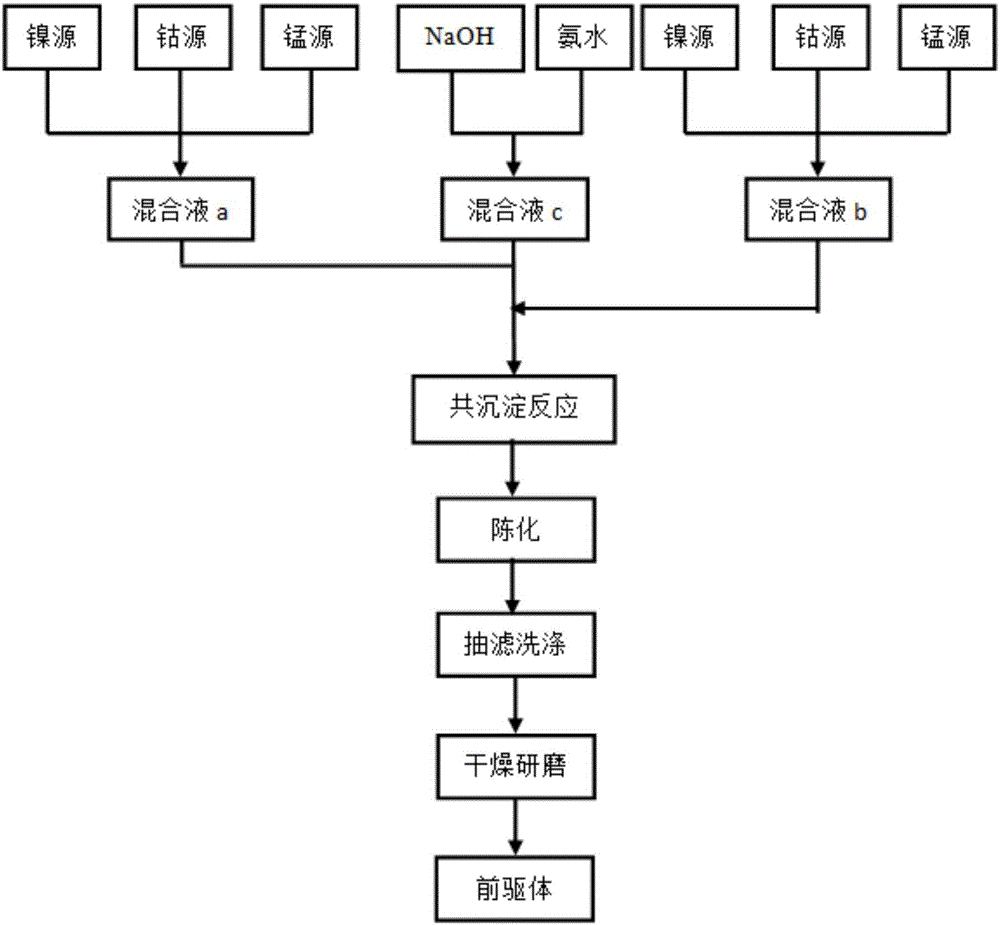
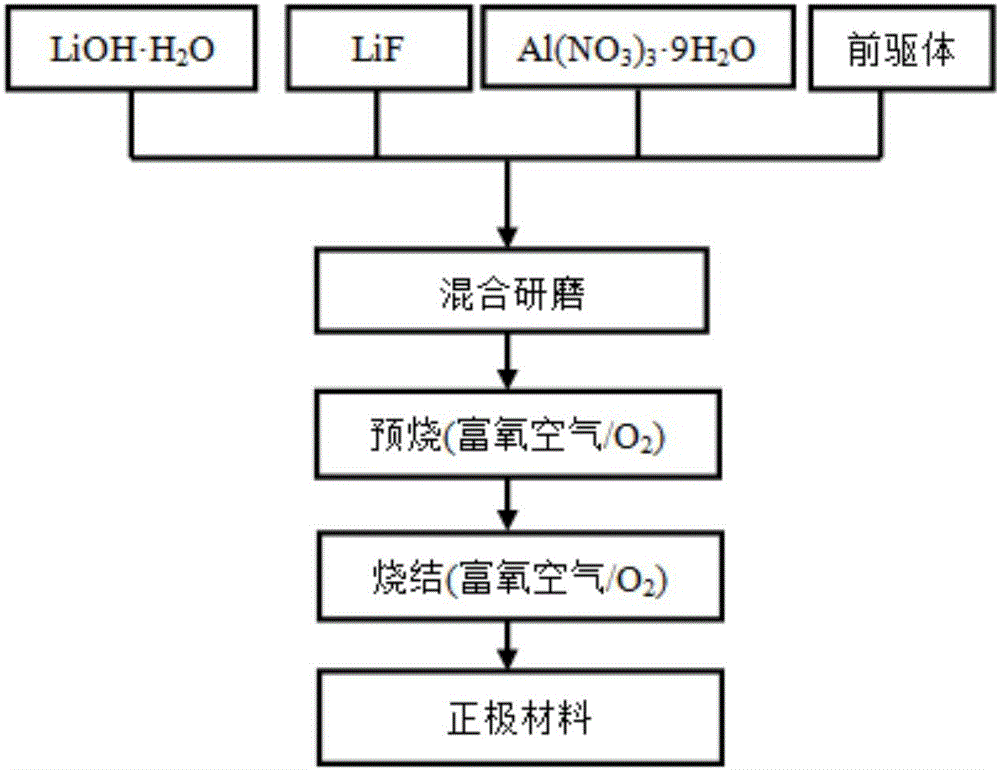
[0034] When Al and F doping amounts are respectively 0.01 and 0.02 (i.e. x=0.01, y=0.02), the nickel source raw material, cobalt source raw material and manganese source raw material are Ni:Co:Mn=6:2 with the total molar ratio: 2 Weigh the sample and divide it into two parts, respectively dissolve it in an appropriate amount of deionized water according to the molar ratio Ni:Co:Mn=5:2:3 and 7:2:1 to prepare a 1mol / L sulfate mixed solution a and b; sodium hydroxide solution and ammonia are mixed to form a mixed solution with a concentration of sodium hydroxide of 2 mol / L and a concentration of ammonia of 0.5 mol / L, which is recorded as solution c; the prepared two solutions a and b are successively mixed with solution c Slowly add it dropwise into the reaction tank with continuous stirring, adjust the pH with ammonia water to control the pH value at about 11, the reaction temperature is 50°C, coprecipitate reaction for 5h, and then age at 70°C for 12h, and then the obtained prod...
Embodiment 2
[0037] When Al and F doping amounts were 0.02 and 0.04 (i.e. x=0.02, y=0.04) respectively, the nickel source raw material, cobalt source raw material and manganese source raw material were Ni:Co:Mn=6:2 with the total molar ratio: 2 Weigh the sample and divide it into two parts, respectively dissolve it in an appropriate amount of deionized water according to the molar ratio Ni:Co:Mn=5:2:3 and 7:2:1 to prepare a 1mol / L sulfate mixed solution a and b; sodium hydroxide solution and ammonia are mixed to form a mixed solution with a concentration of sodium hydroxide of 2 mol / L and a concentration of ammonia of 0.5 mol / L, which is recorded as solution c; the prepared two solutions a and b are successively mixed with solution c Slowly add it dropwise into the reaction tank with continuous stirring, adjust the pH with ammonia water to control the pH value at about 11, the reaction temperature is 50°C, coprecipitate reaction for 5h, and then age at 70°C for 12h, and then the obtained pr...
Embodiment 3
[0040] When Al and F doping amounts were 0.02 and 0.02 (i.e. x=0.02, y=0.02) respectively, the nickel source raw material, cobalt source raw material and manganese source raw material were Ni:Co:Mn=6:2 with the total molar ratio: 2 Weigh the sample and divide it into two parts, respectively dissolve it in an appropriate amount of deionized water according to the molar ratio Ni:Co:Mn=5:2:3 and 7:2:1 to prepare a 1mol / L sulfate mixed solution a and b; sodium hydroxide solution and ammonia are mixed to form a mixed solution with a concentration of sodium hydroxide of 2 mol / L and a concentration of ammonia of 0.5 mol / L, which is recorded as solution c; the prepared two solutions a and b are successively mixed with solution c Slowly add it dropwise into the reaction tank with continuous stirring, adjust the pH with ammonia water to control the pH value at about 11, the reaction temperature is 60°C, coprecipitate reaction for 5h, and then age at 70°C for 12h, and then the obtained pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com