Recombinant anine interferon alpha standard substance, preparation method and potency determination method thereof
A technology of canine interferon and standard product, which is applied in the field of preparation of recombinant interferon standard product, can solve the problems of inconvenient research and production of canine interferon, inappropriate use of canine interferon and the like, and achieves low production cost, high potency, Reference accurate results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Preparation of recombinant canine interferon-α standard
[0039] 1. Preparation of Recombinant Canine Interferon α Protein Solution
[0040] 1.1 Inoculate engineering bacteria BL21 / pET-28α-rCaIFNα on solid LB medium containing ampicillin, culture at 37°C for 16-24 hours, inoculate positive clones into 3ml LB liquid medium containing ampicillin, shake at 37°C Shake culture for 18 hours to obtain the bacterial liquid;
[0041] 1.2 Inoculate the bacterial liquid obtained in step 1.1 into 50ml of culture medium, the volume ratio of the bacterial liquid to the medium is 1:10, culture it on a shaker at 37°C for 18 hours, and the rotation speed is 200r / min, as the fermentation seed bacterial liquid;
[0042]1.3 Add the prepared LB medium into the fermenter, connect the pH probe and the dissolved oxygen probe for in-situ sterilization, the sterilization temperature is 121°C, and the sterilization time is 20 minutes;
[0043] 1.4 Inoculate the fermented seed liquid into a ferm...
Embodiment 2
[0055] Detection of Recombinant Canine Interferon α Standard
[0056] 1. Determination of protein content
[0057] The protein concentration of the purified rCaIFN-α standard was detected by the Lowry method, and the protein content was 0.4 mg / ml.
[0058] 2. SDS-PAGE for purity identification
[0059] The purity of the rCaIFN-α standard substance was identified by SDS-PAGE, and its purity was 97.34%.
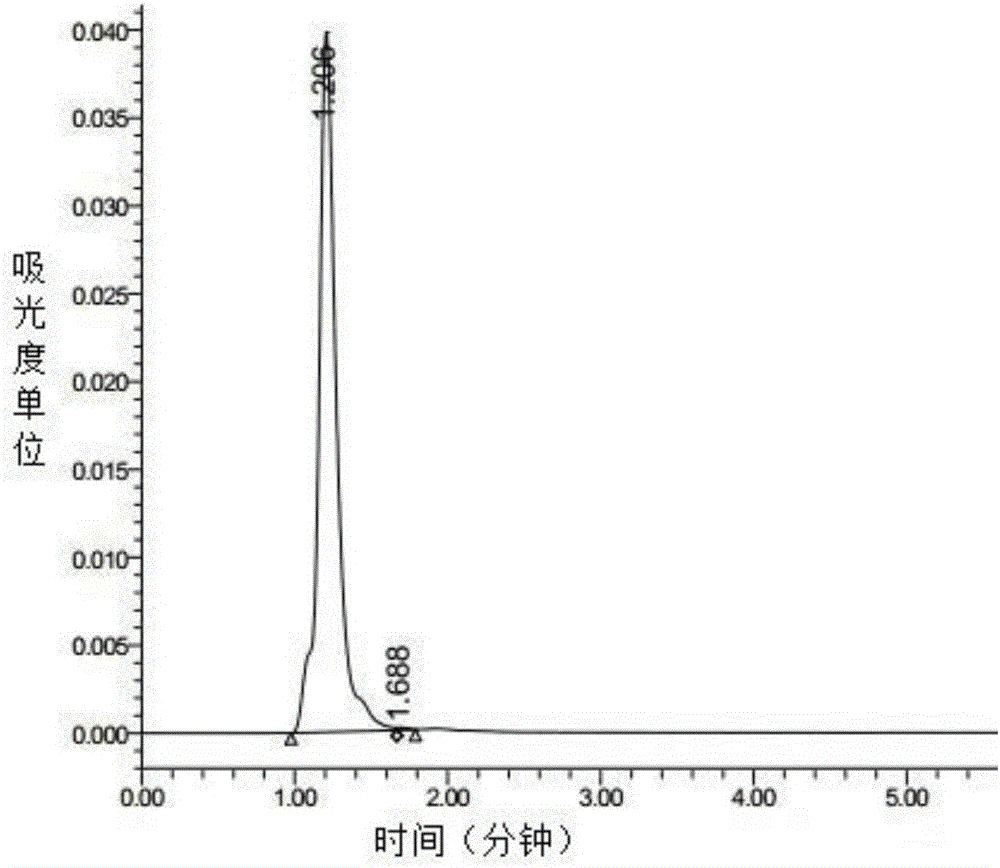
[0060] 3. HPLC purity identification
[0061] The rCaIFN-α standard was analyzed by μRPC C18ST 4.6 / 100 reversed-phase chromatography column and only one main absorption peak was obtained as figure 2 , and one peak is a miscellaneous peak. By calculating the peak area, the main peak area accounts for 99.79% of the total area ( figure 1 ).
[0062] 4. N-terminal amino acid sequence determination
[0063] 4.1 Digestion: Take 200 μg of purified rCaIFN-α protein, add 4 units of recombinant enterokinase, and digest with enzyme digestion buffer at 4°C for 16 hours.
[0064] 4...
Embodiment 3
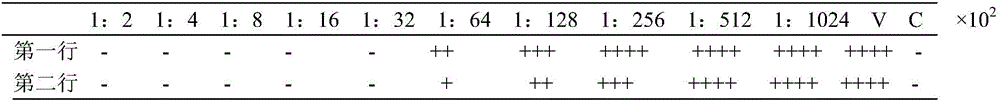
[0070] Potency Determination of Recombinant Canine Interferon α Standard
[0071] This detection method is based on the principle that rCaIFN-α can protect bovine kidney cells (MDBK) from vesicular stomatitis virus (VSV), and inhibits the cytopathic effect (CPE) of the virus with interferon phenomenon as a method to detect its activity. That is, the reciprocal of the dilution that can still protect half of the cells (50%) from virus attack at the highest dilution per milliliter of interferon test product is defined as the interferon unit (or potency), often expressed in units (U), The results were calibrated with national standards. After the result was observed by staining the surviving MDBK cells with crystal violet dye, the potency of the determined interferon was calculated according to the Reed-Munch formula according to the depth of the staining.
[0072] 1. Experimental materials
[0073] Recombinant human interferon α standard: purchased from Beijing China Food and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com