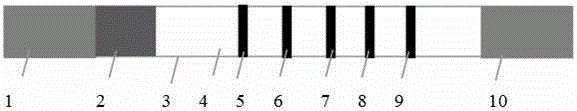
Colloidal gold test strip for detecting four kinds of nitrofuran metabolites and preparation method thereof
A technology of colloidal gold test paper and nitrofuran, which is applied in the direction of biological testing, measuring devices, material inspection products, etc., can solve the problems of too many auxiliary equipment, the inability to achieve combined inspection of nitrofuran metabolites, and high requirements for the experimental environment. The effect of shortening the detection time, promoting the food safety cause, and improving the detection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Measurement and preparation of colloidal gold: add 100mL ultrapure water and 1mL 1% chloroauric acid solution to the round bottom flask in the condensing reflux device, add 1mL 1% trisodium citrate solution after heating to boiling, and observe the color change. When the color is stable, continue to react for 10 minutes, cool and stand for a day, and use a particle size analyzer to obtain colloidal gold with a particle size of 40nm, and its maximum absorption peak is at 526nm.
[0024] 2. Preparation of colloidal gold-labeled antibody: the minimum amount of stable protein should be increased by about 10% for the optimal amount of labeled protein. Colloidal gold with 0.1mol / L of HCl and K 2 CO 3 Adjust the pH to 8.2, and label the antibodies separately. The method is as follows: Take the optimal amount of monoclonal antibody (the antibody labeling amounts of AOZ, AMOZ, AHD, and SEM are: 6 μg / mL, 8 μg / mL, 11 μg / mL, 9 μg / mL) 100mL of colloidal gold solution was added ...
Embodiment 2
[0031] 1. According to 1 in Example 1.
[0032] 2. Preparation of colloidal gold-labeled antibody: the minimum amount of stable protein should be increased by about 10% for the optimal amount of labeled protein. Colloidal gold with 0.1mol / L of HCl and K 2 CO 3 Adjust the pH to 8.2, and label the antibodies separately. The method is as follows: Take the optimal amount of monoclonal antibody (AOZ, AMOZ, AHD, and SEM antibody labeling amounts are: 5μg / mL, 7μg / mL, 9μg / mL, 8μg / mL) 100mL of colloidal gold solution was added to trehalose with a final mass concentration of 1%. After mixing for 10 minutes under a magnetic stirrer, BSA with a final mass concentration of 0.5% was added to obtain 100mL of each of the four gold-labeled antibodies, which were stored overnight at 4°C. Refrigerated ultracentrifugation for purification.
[0033] 3. According to the description in 3 of Example 1.
[0034] 4. According to the description in 4 of Example 1.
[0035] 5. According to the descr...
Embodiment 3
[0039] 1. According to 1 in Example 1.
[0040] 2. Preparation of colloidal gold-labeled antibody: the minimum amount of stable protein should be increased by about 10% for the optimal amount of labeled protein. Colloidal gold with 0.1mol / L of HCl and K 2 CO 3 Adjust the pH to 8.2, and label the antibodies separately. The method is as follows: Take the optimal amount of monoclonal antibody (the antibody labeling amounts of AOZ, AMOZ, AHD, and SEM are: 6 μg / mL, 8 μg / mL, 11 μg / mL, 9 μg / mL) 100mL of colloidal gold solution was added to trehalose with a final mass concentration of 1%. After mixing for 10 minutes under a magnetic stirrer, BSA with a final mass concentration of 0.5% was added to obtain 100mL of each of the four gold-labeled antibodies, which were stored overnight at 4°C. Refrigerated ultracentrifugation for purification.
[0041] 3. According to the description in 3 of Example 1.
[0042] 4. According to the description in 4 of Example 1.
[0043] 5. According to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com