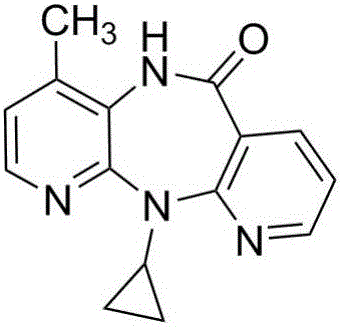
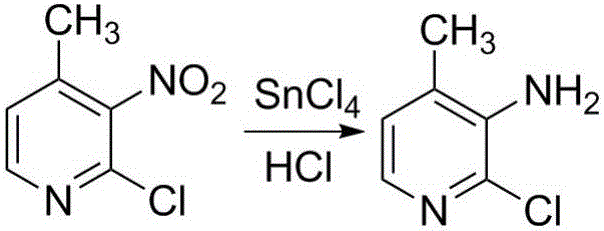
Preparation method for nevirapine intermediate
A kind of methyl pyridine and amino technology, applied in the field of compound preparation, can solve the problems such as shortening synthesis steps, waste acid unfavorable environmental protection, etc., to achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Preparation of 3-bromo-4-methylpyridine
[0069] Add 0.054mol 4-methylpyridine to a 60mL constant pressure dropping funnel; under nitrogen protection and stirring at room temperature, slowly add dropwise to 0.07mol AlCl 3 and 0.01mol potassium bromide, after dropping, continue to stir for 1h; use a condensing device, raise the temperature to 120°C, add 0.07mol bromine dropwise, and dropwise add 0.07mol bromine for about 1h; keep stirring for 26h; the reaction liquid is cooled to At room temperature, the reaction solution was poured into crushed ice under stirring; sodium hydroxide was added, stirred and dissolved; the aqueous layer was extracted with dichloromethane, the organic layers were combined, and the solvent was recovered by rotary evaporation; the obtained oil was subjected to column chromatography (V 石油醚 :V 乙酸乙酯 =6:1) 5.3 g of 3-bromo-4-picoline was obtained as a brownish yellow oil with a purity of 99.9% and a yield of 57%. 1 H NMR (400MHz, CDCl 3 )δ: 2.31...
Embodiment 2
[0071] Preparation of 3-amino-4-picoline
[0072] Prepare 3-amino-4-picoline according to the method described in Example 2 of Chinese patent [CN100999491]: add 300mL methanol, 150g3-bromo-4-picoline, 5g copper sulfate, and feed ammonia into the autoclave When the pressure reaches 5atm, heat to 160°C, react for 8h, cool, filter with suction, and concentrate the filtrate under reduced pressure to obtain a solid that is recrystallized with ethyl acetate to obtain 89g of 3-amino-4-picoline with a yield of 95%.
Embodiment 3
[0074] Preparation of 3-amino-4-picoline
[0075] Prepare 3-amino-4-methylpyridine according to the method described in Example 3 of Chinese patent [CN100999491]: add 500mL concentrated ammonia water, 150g 3-bromo-4-methylpyridine, 5g copper sulfate in the autoclave, and Heated to 180°C, reacted for 8 hours, cooled, extracted three times with 500 mL of dichloromethane, and concentrated under reduced pressure to obtain 84 g of 3-amino-4-picoline with a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com