Combined antitumor drug, preparation method and applications thereof
A technology of anti-tumor drugs and combined drugs, which is applied in the field of anti-tumor drugs and preparations, can solve problems such as rapid release, and achieve the effects of inhibiting proliferation, inhibiting tumor growth, and inhibiting the formation of vascular lumens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
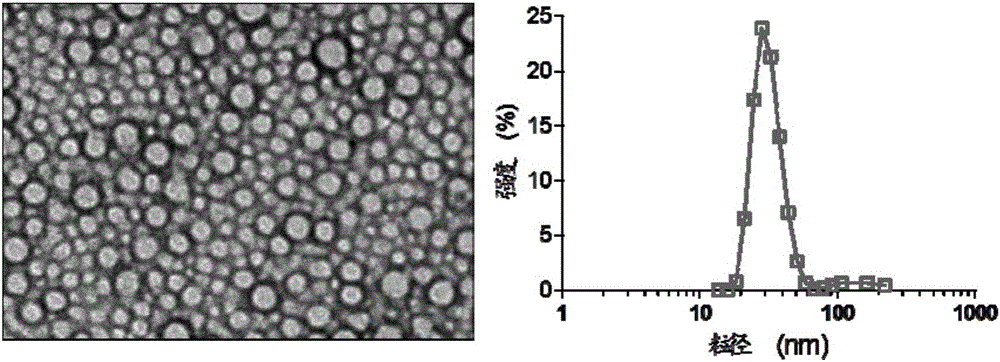
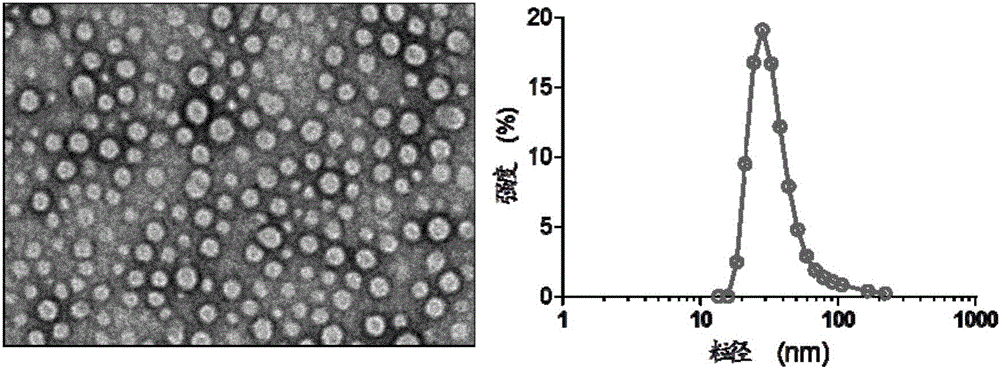
Image
Examples
Embodiment 1
[0043]Dissolve butyric acid (51.7μL, 0.57mmol), CA4 (180mg, 0.57mmol), EDC·HCl (163mg, 0.855mmol), DMAP (76.6mg, 0.627mmol), DIEA (188.9μL, 1.14mmol) in 4ml of DCM , stirred overnight at room temperature, added ethyl acetate, followed by 5% citric acid, saturated NaHCO 3 , saturated brine to wash the organic phase, the organic phase was dried with anhydrous sodium sulfate, filtered, and the solvent was removed under reduced pressure after collecting the filtrate; the solid was separated and purified by column chromatography (ethyl acetate:n-hexane=1:3), to obtain the target The product butyric acid-CA4 conjugate compound 2 (280 mg, yield 93%).
[0044] Product Butyrate-CA4 1 H NMR nuclear magnetic data and mass spectrometry data are as follows:
[0045] 1 H NMR (400MHz, CDCl 3 ):δ1.00-1.04(t,3H),1.73-1.78(m,2H),2.49-2.53(t,2H),3.71(s,6H),3.80(s,3H),3.83(s,3H ),6.45-6.45(d,2H,J=1.2),6.51(s,2H),6.83-6.85(d,1H,J=8.4),7.00-7.01(d,1H,J=1.6),7.10- 7.12(q,1H).
[0046] HR-ESI ...
Embodiment 2
[0048] Heptanoic acid (80.8 μL, 0.57 mmol), CA4 (180 mg, 0.57 mmol), EDC·HCl (163 mg, 0.855 mmol), DMAP (76.6 mg, 0.627 mmol), DIEA (188.9 μL, 1.14 mmol) were dissolved in 4 mL of DCM , stirred overnight at room temperature, added ethyl acetate, followed by 5% citric acid, saturated NaHCO 3 , saturated brine to wash the organic phase, the organic phase was dried with anhydrous sodium sulfate, filtered, and the solvent was removed under reduced pressure after collecting the filtrate; the solid was separated and purified by column chromatography (ethyl acetate:n-hexane=1:3), to obtain the target The product heptanoic acid-CA4 conjugate compound 3 (260 mg, yield 85%).
[0049] Product Enanthate-CA4 1 H NMR nuclear magnetic data and mass spectrometry data are as follows:
[0050] 1 H NMR (400MHz, CDCl 3 ):δ0.88-0.91(t,3H),1.26-1.41(m,6H),1.70-1.74(t,2H),2.51-2.54(t,2H),3.71(s,6H),3.80(s ,3H),3.84(s,3H),6.45-6.45(d,2H,J=1.6),6.51(s,2H),6.83-6.85(d,1H,J=8.4),7.00-7.00(d, 1H, J...
Embodiment 3
[0053] The synthesis method of LNA-SN38 conjugate compound 4 refers to the synthesis of SN38 prodrug 7 in the patent document "7-ethyl-10-hydroxycamptothecin prodrug and its preparation method and application", and the patent application number is 201410295432. X.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com