Cardiac failure resisting medicine LCZ696 oral sustained-release pellets and preparation method thereof
A technology of slow-release pellets and heart failure, applied in the field of medicine, can solve the problems of peak and valley blood drug concentration, easy adhesion to the mouth and throat, adverse drug side effects, etc., achieve low production cost, simple operation, and reduce drug toxicity side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
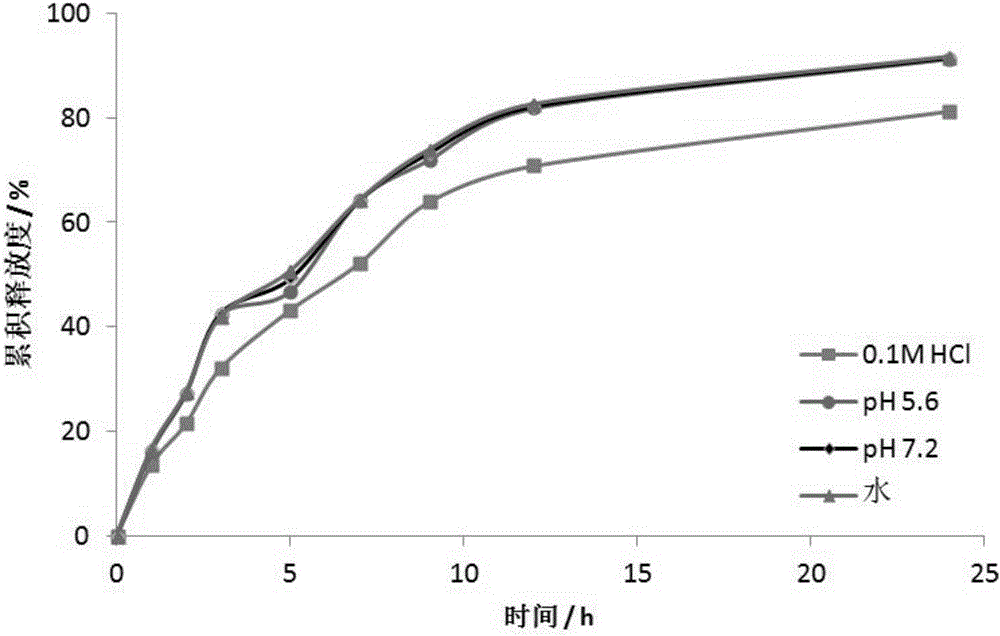
Embodiment 1
[0031] An anti-heart failure drug LCZ696 oral sustained-release pellets:
[0032] Including the core and the coating outside the core, the core ingredients are: LCZ696 2g, microcrystalline cellulose 10g, lactose 5g, hydroxypropyl methylcellulose 1g; the coating ingredients are: Eudragit RS100 2g, PEG4000 0.8g , triethyl citrate 1g, talc powder 1g, titanium dioxide 0.2g.
[0033] The preparation method of the above-mentioned anti-heart failure drug LCZ696 oral sustained-release pellets: take the materials according to the quality of the above-mentioned raw materials, first mix LCZ696 with microcrystalline cellulose and lactose, and use hydroxypropyl methylcellulose as a binder , the centrifugal-fluidization method is used in the centrifugal coating granulator to make the pellet core, and then the pellet core is coated with the coating ingredients in the same way, and after the coating is completed, it is obtained.
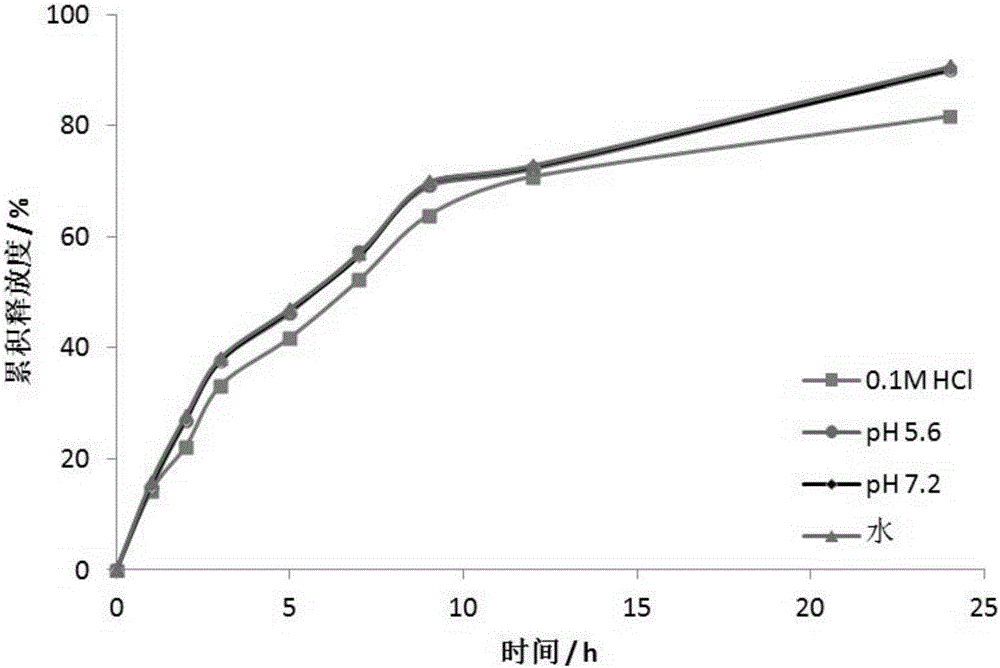
Embodiment 2
[0035] An anti-heart failure drug LCZ696 oral sustained-release pellets:
[0036]Including the ball core and the coating outside the ball core. The core ingredients are: LCZ696 15g, microcrystalline cellulose 15g, mannitol 5g, ethyl cellulose 8g; the coating ingredients are: polyvinylpyrrolidone 5g, Eudragit RL100 3g, PEG4000 0.1g, dimethyl phthalate 0.1g, talc powder 0.1g.
[0037] The preparation method of the above-mentioned anti-heart failure drug LCZ696 oral sustained-release pellets: take the materials according to the quality of the above-mentioned raw materials, first mix LCZ696 with microcrystalline cellulose and mannitol evenly, and use ethyl cellulose as a binder. Roll in a coating pan to form a ball core, then add coating ingredients and continue rolling to coat the ball core. After the coating is completed, it is ready.
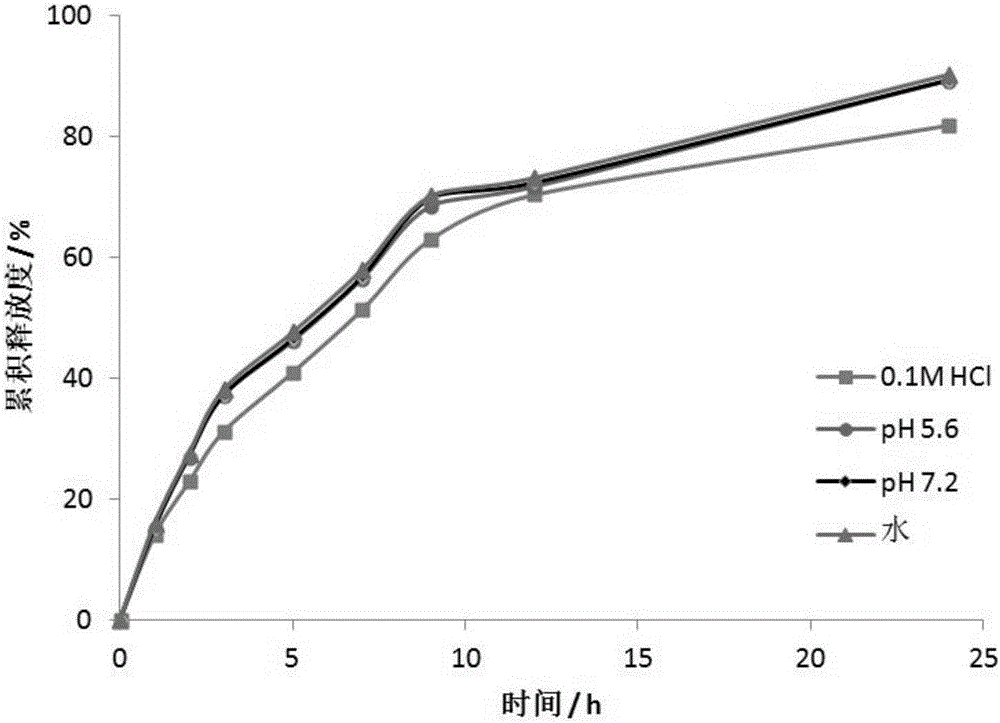
Embodiment 3
[0039] An anti-heart failure drug LCZ696 oral sustained-release pellets:
[0040] Including the core and the coating outside the core, the core ingredients are: LCZ696 35g, sucrose powder 30g, compressible starch 30g, medicinal calcium carbonate 10g, methyl cellulose 6g; the coating ingredients are: Eudragit NE30D 25g , PEG4000 3g. Triethyl citrate 3g, talcum powder 10g, sodium lauryl sulfate 5g.
[0041] The preparation method of the above-mentioned anti-heart failure drug LCZ696 oral sustained-release pellets: take materials according to the quality of the above-mentioned raw materials, first mix LCZ696 with sucrose powder, compressible starch and medicinal calcium carbonate, and methyl cellulose is The binder is made into pellet cores in a extrusion-pellet pellet machine, and then the pellet cores are coated with coating ingredients. After the coating is completed, it is ready to be obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com