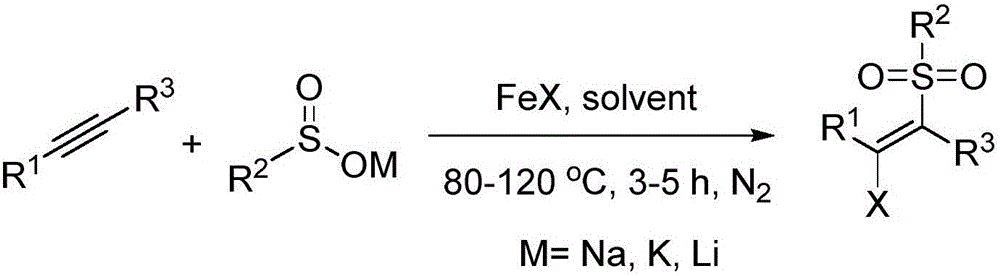
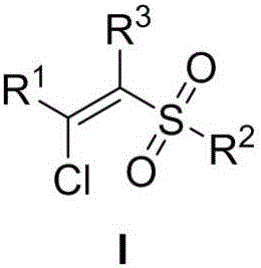
A method of preparing beta-chloroalkenyl sulfone compounds from sulfonates and alkynes
A sulfinate compound and sulfinate technology, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve problems such as long reaction time, expensive ligands, and narrow substrate applicability. Achieve the effects of simple reaction operation, many types of alkynes, and wide applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0031] Synthesis of (E)-1-((2-chloro-2-styryl)sulfonyl)-4-methylbenzene
[0032] Add 0.20mmol phenylacetylene, 0.30mmol sodium p-methylphenylsulfinate, 0.40mmol ferric chloride hexahydrate, and 2.0mL trifluoroethanol solvent into the reactor. Under a nitrogen atmosphere, heat to 80°C, keep stirring for 3 hours, stop the reaction, cool to room temperature, extract with dichloromethane, dry, and distill off the solvent under reduced pressure. The crude product is separated by column chromatography to obtain the target product with a yield of 94%. . 1 H NMR (400MHz, CDCl 3 ): δ7.43(d, J=7.9Hz, 2H), 7.35(d, J=7.1Hz, 1H), 7.32-7.30(m, 3H), 7.28(s, 1H), 7.13(d, J= 7.8Hz,2H),6.85(s,1H),2.32(s,3H).
Synthetic example 2
[0034] Synthesis of (E)-1-((2-chloro-2-(4-methylphenyl)vinyl)sulfonyl)-4-methylbenzene
[0035] Add 0.20mmol p-tolueneacetylene, 0.40mmol lithium p-tolylsulfinate, 0.40mmol ferric chloride hexahydrate, and 2.0mL trifluoroethanol solvent into the reactor. Under argon atmosphere, heat to 100°C, keep stirring for 4h, stop the reaction, cool to room temperature, extract with dichloromethane, dry, and distill off the solvent under reduced pressure, the crude product is separated by column chromatography to obtain the target product, the yield is 76 %. 1 H NMR (400MHz, CDCl 3 ): δ7.47(d, J=8.0Hz, 2H), 7.24(d, J=7.9Hz, 2H), 7.15(d, J=8.0Hz, 2H), 7.10(d, J=7.9Hz, 2H ),6.79(s,1H),2.33(s,3H),2.32(s,3H).
Synthetic example 3
[0037] Synthesis of (E)-1-((2-chloro-2-(4-bromophenyl)vinyl)sulfonyl)-4-methylbenzene
[0038] Add 0.20mmol p-bromophenylacetylene, 0.30mmol potassium p-methylphenylsulfinate, 0.40mmol ferric chloride, 2.40mmol water and 2.0mL ethanol solvent into the reactor. Under nitrogen atmosphere, heat to 130°C, keep stirring for 5h, stop the reaction, cool to room temperature, extract with dichloromethane, dry, and distill off the solvent under reduced pressure, the crude product is separated by column chromatography to obtain the target product, the yield is 75% . 1 H NMR (400MHz, CDCl 3 ): δ7.46(d, J=7.9Hz, 2H), 7.42(d, J=8.1Hz, 2H), 7.19(d, J=8.7Hz, 2H), 7.17(d, J=8.1Hz, 2H ),6.83(s,1H),2.33(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com