Method for preparing ambrotone
A technology of ambergris ketone and methyl group, applied in the field of synthetic perfume, can solve the problems of increasing the difficulty of washing and separation, limiting the scope of use of products, and high equipment requirements, shortening the post-reaction treatment process, improving the scope of application of products, and improving product quality. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
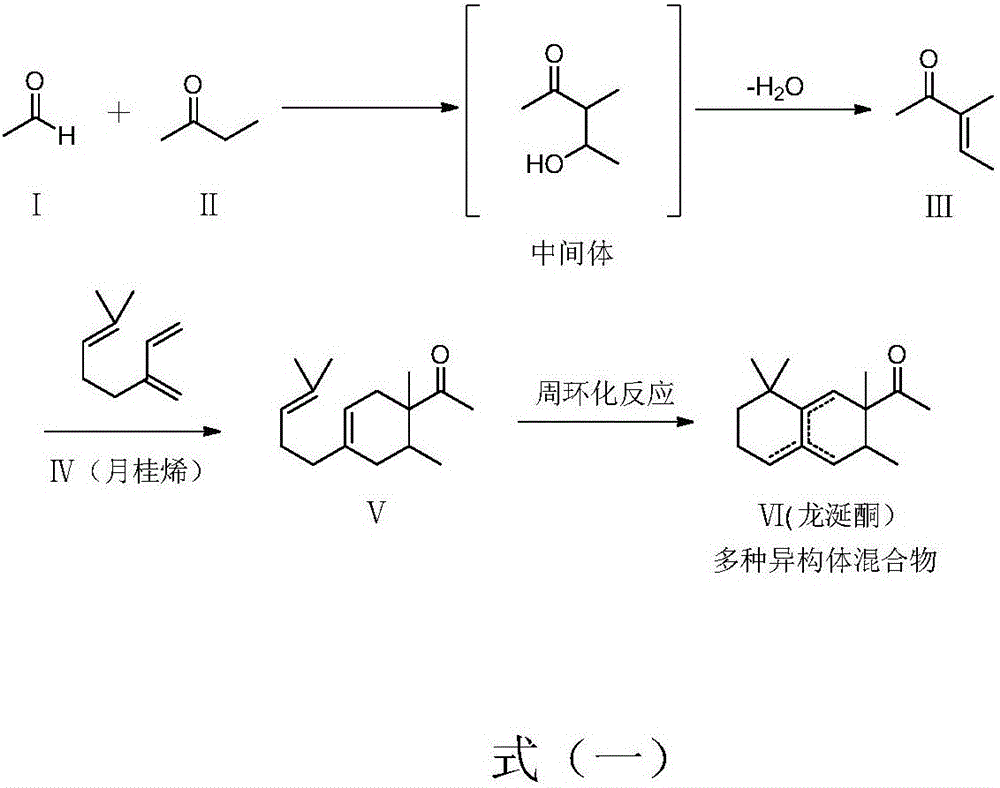
[0040] A method for preparing ambroxol, comprising steps ① synthesizing 3-methyl-3-penten-2 ketone, step ② diene addition to prepare ambroxone intermediate and step ③ cyclization reaction to prepare ambroxone, which It is characterized in that: Step 2. Diene addition to prepare the ambroxone intermediate is completed by high-temperature gas-phase reaction without using a catalyst.
[0041] The present invention preferably following technical scheme:
[0042] Step 2. Diene addition to prepare the ambroxone intermediate is as follows:
[0043] In reaction vessel 1, add β-pinene, then heat to 300-600°C while stirring, crack β-pinene into myrcene, and then directly introduce the cracked myrcene vapor into reaction vessel 3;
[0044] In the reaction vessel two, add the 3-methyl-3-penten-2 ketone synthesized in step ①, atomize or vaporize the 3-methyl-3-penten-2 ketone, and then atomize or vaporize the 3-Methyl-3-penten-2-ketone is introduced into the reaction vessel three, so tha...
Embodiment 1- Embodiment 4
[0065] Embodiment 1-embodiment 4 is the specific example of synthesizing 3-methyl-3-penten-2 ketone
Embodiment 1
[0067] In a 250mL glass jacketed reaction kettle equipped with a reflux condenser, mechanical stirring, thermometer and dropping funnel, butanone (60.0g, 0.833mol) and potassium hydroxide (1.50g, 0.0270mol) were added successively, and the reaction kettle After the temperature was slowly heated to 40°C, 40% acetaldehyde aqueous solution (83.3g, 0.758mol) was added dropwise at this temperature, and the reaction temperature was controlled between 40-45°C. After the dropwise addition, the reaction was carried out at constant temperature for 3 h. After the chromatographic detection reaction was completed, 40 g of ice water was slowly added to the reaction kettle, and neutralized to neutral with concentrated hydrochloric acid. After stirring at room temperature for 30 minutes, the layers were separated. The organic phase was rectified under reduced pressure, and the 68°C / 50mmHg fraction was collected to obtain light yellow liquid compound III (61.1 g, 0.623 mol), with a yield of 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com