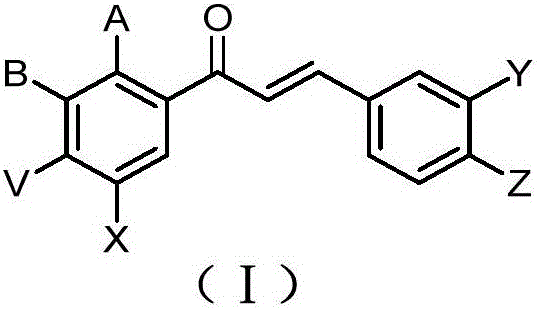
Carbamate-chalcones cholinesterase inhibitors as well as preparation method and application thereof
The technology of carbamate and phenyldimethyl carbamate is applied in the field of carbamate-chalcone cholinesterase inhibitor and preparation thereof, and can solve the problem that the disease state cannot be fundamentally improved or terminating the progression of the disease, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
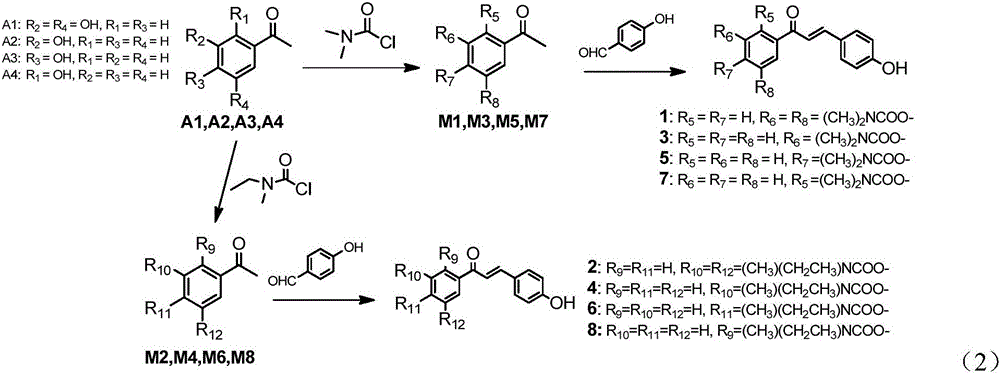
[0149] Preparation of Intermediates (M1-M10):
[0150] 5-Acetyl-1,3-phenylene bis(dimethylcarbamate) (M1)
[0151] 5-Acetyl-1,3-phenylene bis(ethyl(methyl)carbamate) (M2)
[0152] 3-Acetyldimethylcarbamate (M3)
[0153] 3-Acetylethyl (methyl) carbamate (M4)
[0154] 4-Acetyldimethylcarbamate (M5)
[0155] 4-Acetoethyl(methyl)carbamate (M6)
[0156] 2-Acetyldimethylcarbamate (M7)
[0157] 2-Acetoethyl (methyl) carbamate (M8)
[0158] 3-Acetylphenyldimethylcarbamate (M9)
[0159] 3-Acetylisopropylcarbamate (M10)
[0160] General synthesis steps of M1-M10: (0.044mol) hydroxyacetophenone, (0.785eq, 0.035mol) K 2 CO 3 / 1.5H 2 O, (0.215eq, 0.009mol) anhydrous K 2 CO 3 and (0.144eq, 0.0063mol) pyridine in an appropriate amount of ethyl acetate, stirred evenly, and heated to 70°C; (1.5eq, 0.066mol) carbamoyl chloride was stirred evenly in an appropriate amount of ethyl acetate, and added dropwise to the above solution, React at 70°C (the reaction time is determined by mon...
Embodiment 2
[0162] Preparation of intermediates (M11-M14):
[0163] 4-Formylphenyldimethylcarbamate (M11)
[0164] 4-Formylethyl (methyl) carbamate (M12)
[0165] 3-Formylphenyldimethylcarbamate (M13)
[0166] 3-Formylethyl (methyl) carbamate (M14)
[0167] General synthesis steps of M11-M14: (0.044mol) hydroxybenzaldehyde, (0.785eq, 0.035mol) K 2 CO 3 / 1.5H 2 O, (0.215eq, 0.009mol) anhydrous K 2 CO 3 and (0.144eq, 0.0063mol) pyridine in an appropriate amount of ethyl acetate were stirred evenly, and heated to 70°C; (1.5eq, 0.066mol) carbamoyl chloride was stirred evenly in an appropriate amount of ethyl acetate, and added dropwise to the above solution, React at 70°C (the reaction time is determined by monitoring the TLC plate). After the reaction, add water equal to the volume of the reaction solution, and stir at 70°C for 1.5h; cool to room temperature, extract and collect the organic phase, and wash each part with 2wt% sulfuric acid and water. After two times, they were dried ...
Embodiment 3
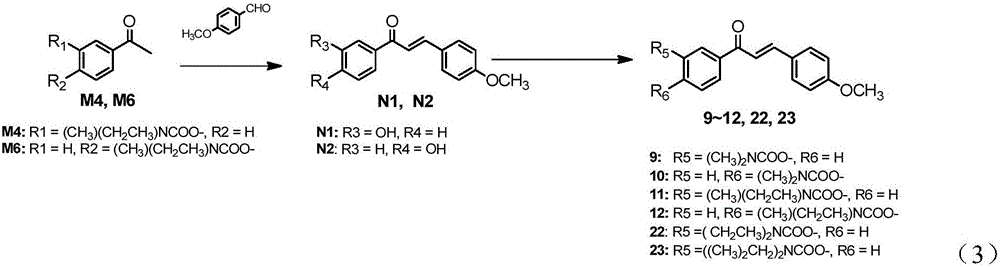
[0169] Synthesis of intermediate (E)-1-(3-hydroxyphenyl)-3-(4-methoxyphenyl)prop-2-en-1-one (compound represented by chemical structural formula N1):
[0170] At room temperature, dissolve (0.4425g, 2mmol) intermediate M4 in 5ml of methanol and stir evenly, drop 4ml of 8wt% KOH solution into it, and dissolve (0.2440g, 3.6mmol) p-methoxybenzaldehyde in methanol Drop into the above solution, stir at room temperature for 72 hours, concentrate in vacuo to remove the solvent, add 90ml of dichloromethane and 90ml of water, collect the organic phase, wash with water three times, dry over anhydrous magnesium sulfate, suction filter, concentrate, and dry to obtain a brown oil, which is washed by Column chromatography (petroleum ether: ethyl acetate = 15:1 ~ 6:1) gave 233.7 mg of light yellow solid product N1 with a yield of 45.9%. M.P.126.5-128.3℃.1H NMR(400MHz,DMSO)δ9.76(s,1H),7.84(d,J=8.7Hz,2H),7.70(s,2H),7.60(d,J=7.7Hz, 1H),7.44(s,1H),7.36(t,J=7.9Hz,1H),7.08–6.97(m,3H),3.83(s,3H).1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com